Methyl Orange
Synonym(s):4-[4-(Dimethylamino)phenylazo]benzenesulfonic acid sodium salt;4-Dimethylaminoazobenzene-4′-sulfonic acid sodium salt, Gold orange, Helianthine, Orange III;Acid Orange 52;Helianthin;Orange III
- CAS NO.:547-58-0
- Empirical Formula: C14H14N3NaO3S
- Molecular Weight: 327.33
- MDL number: MFCD00007502
- EINECS: 208-925-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-31 18:46:13
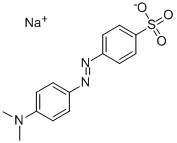
What is Methyl Orange?
Chemical properties
Orange-yellow powder. Soluble in hotwater; insoluble in alcohol.
The Uses of Methyl Orange
Methyl orange is a pH indicator frequently used in titrations, also used for histological microscopy.
The Uses of Methyl Orange
As indicator in 0.1% aqueous solution. pH: 3.1 red, 4.4 yellow. Employed for titrating most mineral acids, strong bases, estimating alkalinity of waters; useless for organic acids. In dyeing and printing of textiles.
What are the applications of Application
Methyl orange is a pH indicator dye for titrations
Definition
An acid–base indicator that is red in solutions below a pH of 3 and yellow above a pH of 4.4. As the transition range is clearly on the acid side, methyl orange is suitable for the titration of an acid with a moderately weak base, such as sodium carbonate.
Definition
methyl orange: An organic dyeused as an acid–base indicator. Itchanges from red below pH 3.1 toyellow above pH 4.4 (at 25°C) and isused for titrations involving weakbases.
Preparation
4-Aminobenzenesulfonic acid diazo, and N,N-dimethylaniline coupling.
General Description
Orange powder.
Air & Water Reactions
Azo dyes can be explosive when suspended in air at specific concentrations. Insoluble in water.
Reactivity Profile
Methyl Orange is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides.
Purification Methods
Recrystallise it twice from hot water, then wash it with a little EtOH followed by diethyl ether. It is an indicator: pH 3.1 (red) and pH 4.4 (yellow). [Beilstein 16 IV 510.]
Properties and Applications
orange. The strong sulfuric acid for green light yellow, diluted into red orange. The dye solution to join strong hydrochloric acid for orange red; Add thick sodium hydroxide solution for green light yellow.
| Standard | Light Fastness | Soaping | Persperation Fastness | Oxygen bleaching | Fastness to seawater | |||
| Fading | Stain | Fading | Stain | Fading | Stain | |||
| ISO | ||||||||
| AATCC | ||||||||
Properties of Methyl Orange
| Melting point: | 300 °C |
| Density | 0.987 g/mL at 25 °C |
| Flash point: | 37 °C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 5g/l |
| form | Powder/Solid |
| Colour Index | 13025 |
| pka | 3.4(at 25℃) |
| color | Yellow-Orange |
| Specific Gravity | 0.987 |
| PH | 6.5 (5g/l, H2O, 20℃) |
| Odor | Odorless |
| PH Range | 3.1(Red)-4.4(Orange) |
| Water Solubility | Soluble in ethanol. Partially soluble in hot water. Slightly soluble in cold water and pyrimidine. Insoluble in ether and alcohol. |
| λmax | 507nm, 522nm, 464nm |
| Merck | 14,6105 |
| BRN | 4732884 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| Biological Applications | Detecting microorganisms; treating dermatological diseases,vaginal affections; dental materials; wound dressing materials |
| Major Application | Liquid crystals, thin films, sensors, sol-gel matrix, waveguides, host-guest chemistry, display device, corrosion inhibitor, glass coatings, paints, wound dressing materials, pharmaceuticals, dental materials, measuring nucleic acid |
| CAS DataBase Reference | 547-58-0 |
| EPA Substance Registry System | Methyl orange (547-58-0) |
Safety information for Methyl Orange
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Methyl Orange
Methyl Orange manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 547 -58 -0 Methyl Orange 98%View Details
547 -58 -0 Methyl Orange 98%View Details
547 -58 -0 -
 Methyl orange CAS 547-58-0View Details
Methyl orange CAS 547-58-0View Details
547-58-0 -
 Methyl Orange CAS 547-58-0View Details
Methyl Orange CAS 547-58-0View Details
547-58-0 -
 Methyl Orange CAS 547-58-0View Details
Methyl Orange CAS 547-58-0View Details
547-58-0 -
 Methyl Orange CAS 547-58-0View Details
Methyl Orange CAS 547-58-0View Details
547-58-0 -
 Methyl Orange 99%View Details
Methyl Orange 99%View Details -
 METHYL ORANGE INDICATOR, Bottles,Drums, PowderView Details
METHYL ORANGE INDICATOR, Bottles,Drums, PowderView Details
547-58-0 -
 5 kg Methyl Orange, PowderView Details
5 kg Methyl Orange, PowderView Details
547-58-0
