Sodium hydroxymethanesulphinate
Synonym(s):Sodium formaldehyde bisulfite
- CAS NO.:149-44-0
- Empirical Formula: CH3NaO3S
- Molecular Weight: 118.09
- MDL number: MFCD00040426
- EINECS: 205-739-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:35
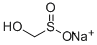
What is Sodium hydroxymethanesulphinate?
Description
Sodium formaldehydesulfoxylate (SFS) is a long name for a rather small molecule. It also goes by the name sodium hydroxymethanesulfinate or, most commonly, its trade name Rongalite. It is usually marketed as the dihydrate.
SFS has a long history:
Today SFS is manufactured from sodium dithionite (Na2S2O4) and formaldehyde. It was originally developed as a treatment for mercury poisoning (hence its pharmaceutical origin), but that was of limited value. Its current uses are in vat dyeing as a reducing agent, in redox polymerization initiator systems, and for aquarium water conditioning.
Earlier this month, Scientific Update, a Mayfield, UK–based firm that holds conferences and training courses for industrial chemists and chemical engineers, featured Rongalite as its Reagent of the Month. The article focuses on the uses of the reagent in organic chemical synthesis.
1. Upjohn is now part of Pfizer. In the May 1949 issue of an Upjohn house publication, Heyl was referred to as “the Father of Upjohn Research”.
2. Later based in West Norfolk, VA. See a 1949 ad in an ACS journal.
Chemical properties
white solid; used as stripping and discharge agent for textiles [HAW93]
Chemical properties
When freshly prepared, sodium formaldehyde sulfoxylate occurs as white, odorless crystals, which quickly develop a characteristic garlic odor on standing.
The Uses of Sodium hydroxymethanesulphinate
Pharmaceutic aid (preservative).
The Uses of Sodium hydroxymethanesulphinate
Sodium Hydroxymethanesulfinate Hydrate is the hydrated version of Sodium Hydroxymethanesulfinate (S634950), which is an organic reductant that is stable in alkaline enviroments but readily decomposes in acidic medium to produce a number of products, one of them being sulfur dioxide. Sodium hydroxymethanesulfinate is also used in conjunction with substituted anilines (e.g. p-Anisidine [A673505]) to synthesize anilinomethanesulfonates.
Production Methods
Sodium formaldehyde sulfoxylate is manufactured from sodium dithionate and formaldehyde in water.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Sodium formaldehyde sulfoxylate is a water-soluble antioxidant and is generally used as the dihydrate. It is used in the formulation of injection products at a level of up to 0.1% w/v in the final preparation administered to the patient.
Safety
The toxicological properties of sodium formaldehyde sulfoxylate
have not been fully investigated. However, it is used in the
formulation of injection products at a level to 0.1% w/v in the
final preparation administered to the patient.
Sodium formaldehyde sulfoxylate is moderately toxic by
ingestion, and when heated to decomposition it emits toxic fumes
of sulfur dioxide and sodium oxide.
LD50 (mouse, oral): 4 g/kg
LD50 (rat, IP): >2 g/kg
LD50 (rat, oral): >2 g/kg
Storage
Store in well-closed, light-resistant containers at controlled room temperature (15–30℃).
Purification Methods
It crystallises from H2O as the dihydrate and decomposes at higher temperatures. Store it in a closed container in a cool place. It is insoluble in EtOH and Et2O and is a good reducing agent. [X-ray structure: Tuter J Chem Soc 3064 1955.] Note that this compound {HOCH2SO2Na} should not be confused with formaldehyde sodium bisulfite adduct {HOCH2SO3Na} from which it is prepared by reduction with Zn. [Beilstein 1 IV 3052.]
Incompatibilities
Sodium formaldehyde sulfoxylate is incompatible with strong oxidizing agents; it is decomposed by dilute acid.
Regulatory Status
Included in the FDA Inactive Ingredients Database (parenteral products up to 0.1% via the IM, IV, and SC routes). Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Sodium hydroxymethanesulphinate
| Melting point: | ~120 °C (dec.) |
| Density | 1.744[at 20℃] |
| vapor pressure | 0.003Pa at 20℃ |
| Flash point: | >100℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | Solid |
| appearance | colorless crystals |
| color | White to Off-White |
| PH | 9.5-10.5 |
| Water Solubility | soluble H2O, alcohol [HAW93] |
| Merck | 14,8620 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | Sodium sulfoxylate formaldehyde (anhydrous) (149-44-0) |
Safety information for Sodium hydroxymethanesulphinate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Sodium hydroxymethanesulphinate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 149-44-0 98%View Details
149-44-0 98%View Details
149-44-0 -
 Sodium Formaldehyde Sulphoxylate 99%View Details
Sodium Formaldehyde Sulphoxylate 99%View Details
149-44-0 -
 Sodium Formaldehyde Sulphoxylate 149-44-0 99%View Details
Sodium Formaldehyde Sulphoxylate 149-44-0 99%View Details
149-44-0 -
 Sodium Formaldehyde Sulfoxylate Hydrate (Rongalite) pure CAS 149-44-0View Details
Sodium Formaldehyde Sulfoxylate Hydrate (Rongalite) pure CAS 149-44-0View Details
149-44-0 -
 Rongalite 95% CAS 149-44-0View Details
Rongalite 95% CAS 149-44-0View Details
149-44-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
