Methyl chloroformate
- CAS NO.:79-22-1
- Empirical Formula: C2H3ClO2
- Molecular Weight: 94.5
- MDL number: MFCD00000639
- EINECS: 201-187-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
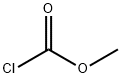
What is Methyl chloroformate?
Description
Methyl chloroformate is a colorless liquidwith an unpleasant, acrid odor. This is a highly corrosiveandflammablematerial. Molecular weight = 94.50;Specific gravity (H2O:1)= 1.22; Boiling point= 71℃;Freezing/Melting point=- -61℃; Flash point= 17℃;Autoignition temperature = 504℃. Flammability Limits:LEL= 6.7%; UEL = unknown.Hazard Identification(based on NFPA-704 M Rating System): Health 1,Flammability 3, Reactivity 1. Slightly soluble in water.
Chemical properties
Methyl chloroformate is a colorless liquid with an unpleasant, acrid odor. This is a highly corrosive and flammable material. Decomposed by hot water; stable to cold water. Soluble in methanol alcohol, ether, and benzene.
The Uses of Methyl chloroformate
Chloroformic Acid Methyl Ester is used in the synthesis of a new class of potent Cdk4 inhibitors in the treatment of cancer. Also used in the synthesis of Phorboxazole B.
Production Methods
Prepared from phosgene and methyl alcohol.
What are the applications of Application
Methyl chloroformate (MCF) is generally used for the derivatization of functional groups such as carboxylic acids, amines, and phenols.
MCF can also be used:
To activate 3-acylpyridines for nucleophilic addition with alkynyltin reagents to form 2,3-disubstituted 1,2-dihydropyridines.
Chloroesterification of terminal alkynes to form β-chloro-α,β-unsaturated esters.
As an electrophilic reagent to mediate the reaction of pyridine with lithium dialkyl- or diarylcuprates to form 4-substituted 1,4-dihydropyridine derivatives.
To convert nitronates into methoxycarbonyl nitronates.
General Description
A colorless liquid with a pungent odor. Flash point 54°F. Corrosive to metals and tissue. Vapors heavier than air. Very toxic by inhalation. Used to make other chemicals and insecticides.
Air & Water Reactions
Highly flammable. Gives off hydrochloric acid fumes in contact with moist air. Slightly soluble in water and decomposed by water to hydrochloric acid with evolution of heat.
Reactivity Profile
Methyl chloroformate is incompatible with water, strong oxidizing agents, alcohols, bases (including amines). Decomposes slowly in water to yield methanol, HCl, and CO2; reaction can be hazardous if water is hot. Attacks many metals especially in humid atmosphere [Handling Chemicals Safely 1980. p. 476]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Flammable, dangerous fire risk. Highly corrosive and irritant to skin and eyes.
Health Hazard
Methyl chloroformate is highly toxic upon inhalation and upon ingestion. A concentration of 1 mg/liter (190 ppm) has been lethal in 10 minutes. It is corrosive and irritating to skin.
Fire Hazard
Methyl chloroformate is very dangerous when exposed to heat sources, sparks, flame, or oxidizers. Methyl chloroformate will react with water or steam to produce toxic and corrosive fumes. Vapors may travel to a source of ignition and flash back. Withdraw immediately in case of rising sound from venting safety device or any discoloration of tank due to fire. Toxic fumes of phosgene are produced when the material is heated to decomposition. Heat or steam should be avoided.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water: Reacts slowly, evolving hydrogen chloride (hydrochloric acid). Reaction can be hazardous if water is hot; Reactivity with Common Materials: Corrodes rubber; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water, rinse with sodium bicarbonate or lime solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Poison by ingestion, inhalation, and intraperitoneal routes. Moderately toxic by skin contact. Human systemic effects by inhalation: conjunctiva irritation and respiratory effects. Corrosive to skin, eyes, and mucous membranes. Very dangerous fire hazard when exposed to heat sources, sparks, flame, or oxidzers. Reacts with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits toxic fumes of Cl-, methyl chloroformate, and phosgene.
Synthesis
Methyl chloroformate prepared by reacting methyl alcohol with phosgene.
A side product (methyl carbonate) could be formed if the temperature is not kept rather low and phosgene is not used in excess:
To a reaction flask fitted with a dropping-funnel, a tube to introduce the gaseous phosgene and an exit tube a 10 ml of methyl chloroformate is placed. The reaction flask is cooled to 0° C and the current of phosgene containing no chlorine is bubbled in. About one-third of reaction flask is filled with methanol from the dropping-funnel all at once. When the phosgene gas is no longer being absorbed additional portion of a fresh methanol is added to the reaction mixture. As soon as the reaction is complete, methyl chloroformate is transferred to a separatory funnel containing cold water. The heavier layer which separates from the aqueous layer is washed twice with cold water, dried over calcium chloride and fractionally distilled. The fraction passing over between 69-72 °C is collected. The yield of methyl chloroformate is about 70% of theory.
The war gases chemistry and analysis, by M. Sartory, 102, 1939.
Potential Exposure
Used in synthesis of pharmaceuticals; herbicides, plastics and other organic chemicals; as a solvent in the photographic industry; as a chemical intermediate in the production of other chemicals. In WWI it was used as military tear-producing warfare agent.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifing upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contarminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24- 48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmo-nary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with methyl chloroformate you should betrained on its proper handling and storage. Before enteringconfined space where this chemical may be present, checkto make sure that an explosive concentration does not exist.Store in tightly closed containers in a cool, well-ventilatedarea away from all forms of moisture, oxidizers, strongacids, strong bases. See incompatibilities for other materialsto avoid. Where possible, automatically pump liquid fromdrums or other storage containers to process containers.Drums must be equipped with self-closing valves, pressurevacuum bungs, and flame arresters. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of this chemical. Wherever this chemical is used,handled, manufactured, or stored, use explosion-proof electrical equipment and fittings.
Shipping
UN1238 Methyl chloroformate, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, 8-Corrosive material Inhalation Hazard Zone A
Incompatibilities
May form explosive mixture with air. Violent reaction with alkali metals; ethers. Incompatible with strong acids; strong bases; alcohols, oxidizers, dimethylsulfoxide; dimethyl formamide. Contact with water or moisture produces corrosive and poisonous hydrogen chloride gas, methanol and carbon monoxide. Corrodes metals in the presence of moisture. Attacks some plastics, rubber and coatings.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal
Properties of Methyl chloroformate
| Melting point: | -61°C |
| Boiling point: | 70-72 °C(lit.) |
| Density | 1.223 g/mL at 25 °C(lit.) |
| vapor density | 3.26 (vs air) |
| vapor pressure | 4.8 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 64 °F |
| storage temp. | Store at 0-5°C |
| solubility | Chloroform, Ethyl Acetate |
| form | Liquid |
| color | Clear colorless to light yellow |
| Odor | Acrid. |
| Water Solubility | hydrolysis |
| Merck | 13,6071 |
| Dielectric constant | 12.9 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 79-22-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Carbonochloridic acid, methyl ester(79-22-1) |
| EPA Substance Registry System | Methyl chlorocarbonate (79-22-1) |
Safety information for Methyl chloroformate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H312:Acute toxicity,dermal H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl chloroformate
Methyl chloroformate manufacturer
ASM Organics
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 Methyl chloro formate CAS 79-22-1View Details
Methyl chloro formate CAS 79-22-1View Details
79-22-1 -
 Methyl chloroformate CAS 79-22-1View Details
Methyl chloroformate CAS 79-22-1View Details
79-22-1 -
 Methyl chloroformate, 97% CAS 79-22-1View Details
Methyl chloroformate, 97% CAS 79-22-1View Details
79-22-1 -
 Methyl Chloroformate CASView Details
Methyl Chloroformate CASView Details -
 Methyl chloroformate 98% CAS 79-22-1View Details
Methyl chloroformate 98% CAS 79-22-1View Details
79-22-1 -
 96%Methyl Chloroformate Liquid CAS 79-22-1View Details
96%Methyl Chloroformate Liquid CAS 79-22-1View Details
79-22-1 -
 METHYL CHLOROFORMATE For Synthesis CAS 79-22-1View Details
METHYL CHLOROFORMATE For Synthesis CAS 79-22-1View Details
79-22-1 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
