Ethyl chloroacetate
Synonym(s):Chloroacetic acid ethyl ester;Ethyl chloroacetate
- CAS NO.:105-39-5
- Empirical Formula: C4H7ClO2
- Molecular Weight: 122.55
- MDL number: MFCD00000932
- EINECS: 203-294-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
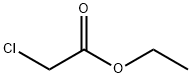
What is Ethyl chloroacetate?
Description
Ethyl chloroacetate is a water-white liquidwith a pungent, fruity odor. Molecular weight = 122.56;Boiling point = 145℃. Freezing point = - 27℃; Flashpoint = 64℃. Hazard Identification (based on NFPA-704 MRating System): Health: 3, Flammability 3, Reactivity 0.Insoluble in water.
Chemical properties
Ethyl chloroacetate is a colourless liquid with a pungent, fruity odor.Ethyl chloroacetate has a vapor pressure of 10mmHg at 38 °C (Lewis, 1997). It is insoluble in water, but miscible with alcohol, ether, and acetone (Lide, 1998); it is soluble in benzene (Lewis, 1997). Ethyl chloroacetate readily decomposes in hot water and alkalis (Lewis, 1997).
The Uses of Ethyl chloroacetate
Ethyl chloroacetate is a reagent used in the preparation of 5 member heterocycles. It is used as pharmaceutical and organic intermediate. It is used as a solvent for organic synthesis and the production of pesticides (such as sodium fluoroacetate).
What are the applications of Application
Ethyl chloroacetate is a reagent used in the preparation of 5 member heterocycles
Preparation
Ethyl chloroacetate is synthesized by esterification of chloroacetic acid and ethanol under the catalysis of sulfuric acid. The reaction equation is as follows:
ClCH2COOH+C2H5OH[H2SO4]→ClCH2COOC2H5+H2O
Reaction: Add chloroacetic acid, ethanol and benzene into the esterification pot, turn on the stirrer, slowly add sulfuric acid, heat to reflux, continuously steam out the benzene-water azeotrope, and de-esterify the water generated by condensation and separator layering, Benzene is refluxed into the esterification pot, cooled and discharged when there is no more water to steam out, the crude ester is washed with saturated sodium bicarbonate solution and water to neutrality, dried with anhydrous calcium chloride, distilled, and collected The fraction at 144-146°C is the finished product of ethyl chloroacetate, the content is ≥99.0%, and the yield is 85%.
Synthesis Reference(s)
Tetrahedron, 23, p. 359, 1967 DOI: 10.1016/S0040-4020(01)83321-4
General Description
A clear colorless liquid with a pungent odor. Flash point 100°F. Denser than water and insoluble in water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Slow hydrolysis to acidic products will cause slow corrosion of common metals. No hazard involved. [USCG, 1999].
Reactivity Profile
Ethyl chloroacetate is a chlorinated ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Hazard
Strong irritant to eyes.
Health Hazard
Inhalation causes irritation of mucous membrane, headache, and nausea. Contact with liquid causes extreme eye irritation and conjunctivitis; irritates skin if not removed at once. Ingestion causes irritation of mouth and stomach.
Fire Hazard
Special Hazards of Combustion Products: Irritating, toxic hydrogen chloride and phosgene may be generated in fires.
Chemical Reactivity
Reactivity with Water Very slow, not hazardous; Reactivity with Common Materials: Slow hydrolysis to acidic products; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
oison by skin contact and subcutaneous routes. A severe eye irritant. Questionable carcinogen with experimental neoplastigenic data.Flammable liquid; a dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Will react with water or steam to produce toxic and corrosive fumes. Vigorous reaction with sodium cyanide. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of cl-.
Potential Exposure
Used to make rodenticides, dyes, and other chemicals. Also used as a military poison
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24- 48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray
storage
(1) Color Code—Red: Flammability Hazard: Storein a flammable liquid storage area or approved cabinetaway from ignition sources and corrosive and reactivematerials. (2) Color Code—Blue: Health Hazard/Poison:Store in a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from oxidizers, strong acids, strongbases, reducing agents, heat, and sources of ignition. Wherepossible, automatically pump liquid from drums or otherstorage containers to process containers. Drums must beequipped with self-closing valves, pressure vacuum bungs,and flame arresters. Use only nonsparking tools and equipment, especially when opening and closing containers ofthis chemical. Sources of ignition, such as smoking andopen flames, are prohibited where this chemical is used,handled, or stored in a manner that could create a potentialfire or explosion hazard. Wherever this chemical is used,handled, manufactured, or stored, use explosion-proof electrical equipment and fittings.
Shipping
UN1181 Ethylchloroacetate, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid.
Purification Methods
Shake the ester with satutated aqueous Na2CO3 (three times), aqueous 50% CaCl2 (three times) and saturated aqueous NaCl (twice). Dry it with Na2SO4 or MgSO4 and distil it. [Beilstein 2 IV 481.] LACHRYMATORY.
Incompatibilities
May form explosive mixture with air. Incompatible with strong bases; strong acids; reducing agents. Moisture, water, and steam contact forms toxic and corrosive fumes. Violent reaction with oxidizers, alkaline earth metals (barium, calcium, magnesium, strontium, etc.), alkaline metals, sodium cyanide. Attacks metals in the presence of moisture.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of Ethyl chloroacetate
| Melting point: | -26 °C (lit.) |
| Boiling point: | 143 °C (lit.) |
| Density | 1.145 g/mL at 25 °C (lit.) |
| vapor density | 4.23 (vs air) |
| vapor pressure | 10 mm Hg ( 38 °C) |
| refractive index | n |
| Flash point: | 150 °F |
| storage temp. | Store below +30°C. |
| solubility | 12.3g/l |
| form | Liquid |
| color | Clear colorless |
| Odor | Extremely irritating; fruity; pungent |
| explosive limit | 2.6%(V) |
| Water Solubility | 20 g/L (20 ºC) |
| Merck | 14,3783 |
| BRN | 506455 |
| Dielectric constant | 11.6(20℃) |
| Stability: | Stable. Incompatible with acids, bases, oxidizing agents, reducing agents. Flammable. |
| CAS DataBase Reference | 105-39-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethyl chloroacetate(105-39-5) |
| EPA Substance Registry System | Ethyl chloroacetate (105-39-5) |
Safety information for Ethyl chloroacetate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H318:Serious eye damage/eye irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ethyl chloroacetate
Ethyl chloroacetate manufacturer
JSK Chemicals
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 Ethyl Chloroacetate pure CAS 105-39-5View Details
Ethyl Chloroacetate pure CAS 105-39-5View Details
105-39-5 -
 Ethyl Chloroacetate CAS 105-39-5View Details
Ethyl Chloroacetate CAS 105-39-5View Details
105-39-5 -
 Ethyl chloroacetate CAS 105-39-5View Details
Ethyl chloroacetate CAS 105-39-5View Details
105-39-5 -
 Ethyl chloroacetate CAS 105-39-5View Details
Ethyl chloroacetate CAS 105-39-5View Details
105-39-5 -
 Ethyl chloroacetate CAS 105-39-5View Details
Ethyl chloroacetate CAS 105-39-5View Details
105-39-5 -
 ETHYL CHLOROACETATE For Synthesis CAS 105-39-5View Details
ETHYL CHLOROACETATE For Synthesis CAS 105-39-5View Details
105-39-5 -
 Ethyl chloroacetate 99% CAS 105-39-5View Details
Ethyl chloroacetate 99% CAS 105-39-5View Details
105-39-5 -
 Ethyl chloroacetate CAS 105-39-5View Details
Ethyl chloroacetate CAS 105-39-5View Details
105-39-5
