Methyl chloroacetate
Synonym(s):Chloroacetic acid methyl ester;Methyl chloroacetate
- CAS NO.:96-34-4
- Empirical Formula: C3H5ClO2
- Molecular Weight: 108.52
- MDL number: MFCD00000931
- EINECS: 202-501-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
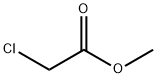
What is Methyl chloroacetate?
Chemical properties
clear colorless liquid with a slight irritating odor. It is miscible with organic solvents such as ethanol, ether, acetone and benzene. Slightly soluble in water.
The Uses of Methyl chloroacetate
Methyl chloroacetate is used as a solvent and chemical intermediate. It acts as a precursor in the preparation of (carboxymethyl) trimethylammonium chloride esters. Further, it is used in the preparation of octakis-(carbethoxymethoxy)calix[8]arene. It is employed as an extraction solvent during the separation of neutral compounds. In addition to this, it is used in the synthesis of dimethyl carbonate.
What are the applications of Application
Methyl chloroacetate (MC) is a halogenated ester mainly used as a solvent in organic synthesis or in the preparation of several compounds. Typically, MC is used in the preparation of (carboxymethyl) trimethylammonium chloride estersor in the synthesis of octakis- (carbethoxymethoxy)calix[8]arene. Additionally, methyl chloroacetate acts as an extraction solvent during the separation of neutral compounds with concentration enhancement using coupling liquid–liquid semi-microextraction with micellar electrokinetic chromatography through oncapillary decomposition.
Preparation
Methyl chloroacetate is prepared by esterification of chloroacetic acid with methanol.
Reaction: Methanol and chloroacetic acid are uniformly mixed in a weight ratio of 0.366:1, heated with stirring, and the esterification reaction is carried out at 105-110 °C. In the reaction process, the ternary azeotrope of methyl chloroacetate, water and methanol is continuously steamed, layered through the ester separator, the separated methanol and water are returned to the reaction pot, and the separated crude ester is made of sodium carbonate. neutralize. The neutralized crude ester is firstly cut out the 130°C fraction by atmospheric distillation, and then subjected to vacuum distillation to collect the 65°C (8kPa) fraction, which is the finished product of methyl chloroacetate. The yield is about 96%.
Synthesis Reference(s)
The Journal of Organic Chemistry, 50, p. 3408, 1985 DOI: 10.1021/jo00218a034
General Description
A crystalline solid or a solid dissolved in a liquid. Insoluble in water and denser than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Methyl chloroacetate is a halogenated ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Hazard
Toxic by ingestion and inhalation.
Health Hazard
Extremely corrosive to the eyes, skin, nose, throat, and upper respiratory tract. Inhalation may be fatal as a result of spasm, inflammation and edema of the larynx and bronchi, chemical pneumonitis, and pulmonary edema. Symptoms of exposure include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting.
Fire Hazard
Special Hazards of Combustion Products: Toxic fumes of hydrogen chloride
Safety Profile
Poison by ingestion. Moderately toxic by inhalation and subcutaneous routes. Flammable when exposed to heat or flame; can react vigorously with oxidizing materials. When heated to decomposition it emits toxic fumes of Cl-.
Purification Methods
Shake the ester with saturated aqueous Na2CO3 (three times), aqueous 50% CaCl2 (three times), saturated aqueous NaCl (twice), dry (Na2SO4) and fractionally distil it. Very toxic. [Beilstein 2 IV 480.]
Properties of Methyl chloroacetate
| Melting point: | -33 °C |
| Boiling point: | 130 °C740 mm Hg(lit.) |
| Density | 1.238 g/mL at 25 °C(lit.) |
| vapor density | 3.8 (vs air) |
| vapor pressure | 10 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 125 °F |
| storage temp. | Store at <= 20°C. |
| solubility | 51.6g/l (slow decomposition) |
| form | Liquid |
| color | Clear colorless |
| PH | 4.6 (28g/l, H2O, 20℃) |
| explosive limit | 4.6-18.5%(V) |
| Water Solubility | 28 g/L (20 ºC) |
| Merck | 14,6042 |
| BRN | 506255 |
| Dielectric constant | 12.9(20℃) |
| CAS DataBase Reference | 96-34-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, chloro-, methyl ester(96-34-4) |
| EPA Substance Registry System | Acetic acid, 2-chloro-, methyl ester (96-34-4) |
Safety information for Methyl chloroacetate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl chloroacetate
Methyl chloroacetate manufacturer
JSK Chemicals
TRUUCHEM TECHNOLOGIES PRIVATE LIMITED
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran



You may like
-
 Methyl chloroacetate 99%View Details
Methyl chloroacetate 99%View Details -
 96-34-4 Methyl chloroacetate 98%View Details
96-34-4 Methyl chloroacetate 98%View Details
96-34-4 -
 Methyl chloroacetate CAS 96-34-4View Details
Methyl chloroacetate CAS 96-34-4View Details
96-34-4 -
 Methyl chloroacetate CAS 96-34-4View Details
Methyl chloroacetate CAS 96-34-4View Details
96-34-4 -
 Methyl chloroacetate CAS 96-34-4View Details
Methyl chloroacetate CAS 96-34-4View Details
96-34-4 -
 Methyl Chloro Acetate CASView Details
Methyl Chloro Acetate CASView Details -
 Methyl chloroacetate CAS 96-34-4View Details
Methyl chloroacetate CAS 96-34-4View Details
96-34-4 -
 Methyl chloroacetate CAS 96-34-4View Details
Methyl chloroacetate CAS 96-34-4View Details
96-34-4
