Methyl bromoacetate
Synonym(s):Bromoacetic acid methyl ester;METHYL BROMOACETATE
- CAS NO.:96-32-2
- Empirical Formula: C3H5BrO2
- Molecular Weight: 152.97
- MDL number: MFCD00000189
- EINECS: 202-499-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
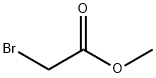
What is Methyl bromoacetate?
Chemical properties
Colorless liquid
The Uses of Methyl bromoacetate
Methyl bromoacetate was used in the synthesis of novel coumarins. It was also employed in the synthesis of cis-cyclopropanes
The Uses of Methyl bromoacetate
Methyl bromoacetate is used in the synthesis of coumarins and cis-cyclopropanes. It reacts with the conjugate base of (methylmethoxycarbene)pentacarbonylchromium(0) to prepare alkylated carbene complexes. Further, it is used to make vitamins and pharmaceuticals.
What are the applications of Application
Methyl bromoacetate is An α-bromo ester.
What are the applications of Application
Methyl Bromoacetate is an organic building block that has been used as a reactant in the preparation of isoquinolinone indole acetic acid derivatives as antagonists of chemoattractant receptor homologous molecule expressed on Th2 cells (CRTH2) for the treatment of allergic inflammatory diseases.
General Description
A colorless to straw-colored liquid with a sharp penetrating odor. Denser than water and soluble in water. Severly irritates skin and eyes. Toxic by ingestion and inhalation. Used to make vitamins and pharmaceuticals.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
Methyl bromoacetate is a halogenated ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Hazard
Vapor is strong irritant to eyes.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Bromoacetates and chloroacetates are extremely irritating/lachrymators. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of Br-. See also ESTERS.
Properties of Methyl bromoacetate
| Melting point: | -50°C |
| Boiling point: | 51-52 °C/15 mmHg (lit.) |
| Density | 1.616 g/mL at 25 °C (lit.) |
| vapor pressure | 18 hPa (50 °C) |
| refractive index | n |
| Flash point: | 145 °F |
| storage temp. | Store below +30°C. |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Specific Gravity | 1.416 |
| Water Solubility | Miscible with methanol, ether and acetone. Slightly miscible with water |
| FreezingPoint | <-50℃ |
| BRN | 506256 |
| CAS DataBase Reference | 96-32-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, bromo-, methyl ester(96-32-2) |
| EPA Substance Registry System | Acetic acid, bromo-, methyl ester (96-32-2) |
Safety information for Methyl bromoacetate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl bromoacetate
Methyl bromoacetate manufacturer
JSK Chemicals
ASM Organics
Related products of tetrahydrofuran

You may like
-
 Methyl bromoacetate 96-32-2 98%View Details
Methyl bromoacetate 96-32-2 98%View Details
96-32-2 -
 96-32-2 Methyl bromoacetate 98%View Details
96-32-2 Methyl bromoacetate 98%View Details
96-32-2 -
 Methyl bromoacetate CAS 96-32-2View Details
Methyl bromoacetate CAS 96-32-2View Details
96-32-2 -
 Methyl bromoacetate, 97% CAS 96-32-2View Details
Methyl bromoacetate, 97% CAS 96-32-2View Details
96-32-2 -
 Methyl Bromoacetate CAS 96-32-2View Details
Methyl Bromoacetate CAS 96-32-2View Details
96-32-2 -
 Methyl bromoacetate 98%View Details
Methyl bromoacetate 98%View Details
96-32-2 -
 Methyl bromoacetate 97% CAS 96-32-2View Details
Methyl bromoacetate 97% CAS 96-32-2View Details
96-32-2 -
 Methyl bromoacetate CAS 96-32-2View Details
Methyl bromoacetate CAS 96-32-2View Details
96-32-2