Methocarbamol
Synonym(s):Guaiacol glyceryl ether carbamate;Methocarbamol
- CAS NO.:532-03-6
- Empirical Formula: C11H15NO5
- Molecular Weight: 241.24
- MDL number: MFCD00057662
- EINECS: 208-524-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
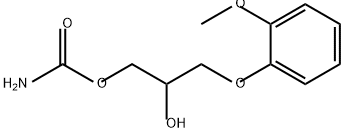
What is Methocarbamol?
Absorption
The time to maximum concentration is 1.1 hours for both healthy patients and those on hemodialysis. The maximum plasma concentration is 21.3mg/L for healthy patients and 28.7mg/L in hemodialysis patients. The area under the curve for healthy patients is 52.5mg/L*hr and 87.1mg/L*hr in hemodialysis patients. AUC% based on terminal elimination half life is 2% for healthy patients and 4% for hemodialysis patients.
Older studies report maximum plasma concentrations in 0.5 hours.
Toxicity
Overdose of methocarbamol may be associated with alcohol and other central nervous system depressants. Patients may experience nausea, drowsiness, blurred vision, hypotension, seizures, and coma. Treatment of overdose is generally through airway maintenance, monitoring urinary output, vital signs, and giving fluid intravenously if necessary.
The oral LD50 in rats is 3576.2mg/kg.
The FDA has classified methocarbamol as pregnancy category C. Animal and human studies have not been performed to determine the risks to a fetus, however fetal and congenital abnormalities have been reported. Methocarbamol is excreted in the milk of dogs, however it is unknown if this is also the case for humans. Caution should be exercised when taking methocarbamol while breastfeeding.
Studies to assess the carcinogenicity, mutagenicity, or effects on fertility of methocarbamol have not been performed.
Chemical properties
White Solid
Originator
Robaxin ,Robins,US,1957
The Uses of Methocarbamol
Methocarbamol suppresses multisynaptic pathways in the spinal cord. It is used for relieving spasms and skeletal muscle pain as well as for treating tetanus. Synonyms of this drug are delaxin, forbaxin, robamol, robaxin, and tresortil.
The Uses of Methocarbamol
A muscle relaxant (skeletal).
The Uses of Methocarbamol
For use as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions.
Background
Methocarbamol was developed in the early 1950s as a treatment for muscle spasticity and the associated pain. It is a guaiacol glyceryl ether.
Methocarbamol tablets and intramuscular injections are prescription medicines indicated in the United States as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. In Canada, methocarbamol can be sold as an over the counter oral medicine at a lower dose that may be combined with acetaminophen or ibuprofen. A combination product with acetylsalicylic acid and codeine is available in Canada by prescription.
Methocarbamol was FDA approved on 16 July 1957.
Indications
Methocarbamol tablets and intramuscular injections are indicated in the United States as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. Oral methocarbamol in America may be given up to 1500mg 4 times daily for 2-3 days.
In Canada, methocarbamol containing oral formulations are sold over the counter for pain associated with muscle spasm. However, if these combination formulations include codeine, they are prescription only.
What are the applications of Application
Methocarbamol is a skeletal muscle relaxant
Definition
ChEBI: 2-hydroxy-3-(2-methoxyphenoxy)propyl carbamate is a carbamate ester that is glycerol in which one of the primary alcohol groups has been converted to its 2-methoxyphenyl ether while the other has been converted to the corresponding carbamate ester. It is a carbamate ester, a secondary alcohol and an aromatic ether.
Manufacturing Process
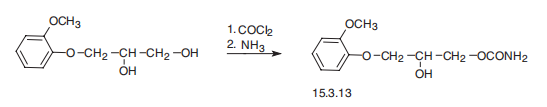
The starting material for methocarbamol is 3-o-methoxyphenoxy-1,2- propanediol (guaiacol glyceryl ether) (see entry under Guaifenesin for its preparation). To a stirred suspension of 198.2 g (1.0 mol) of 3-omethoxyphenoxy-1,2-propanediol in 1,000 ml of dry benzene contained in a 5-liter, 3-neck, round bottom flask equipped with a thermometer, dropping funnel and blade stirrer, was added dropwise (in 30 minutes) a solution of 98.9 g (1.0 mol) of phosgene in 400 ml of cold dry benzene. The mixture was stirred at 30°C until all solid material dissolved (about 3 hours was required) and stirring was continued for 30 minutes longer. To this mixture was added
dropwise 79.1 g (1.0 mol) of dry pyridine, the temperature being held below
30°C by cooling. After addition of the pyridine, stirring at 30°C was continued
for 30 minutes.
The mixture was cooled to 7°C, extracted with two 500-cc portions of ice
water to remove pyridine hydrochloride, and the benzene solution of 3-omethoxyphenoxy-2-hydroxypropyl chlorocarbonate was added to 500 ml of
cold concentrated ammonium hydroxide. The mixture was vigorously stirred at
5°C for 6 hours, then the crude white precipitate of 3-o-methoxyphenoxy-2-
hydroxypropyl carbamate was filtered off, dissolved in 1,500 ml of hot
benzene and completely dried by codistillation of last traces of water with
benzene, treated with decolorizing carbon and filtered while hot. On cooling
160 g of product crystallized as white needles melting at 88° to 90°C.
brand name
Delaxin (Ferndale); Forbaxin (Forest); Robaxin (Baxter Healthcare).
Therapeutic Function
Muscle relaxant
Synthesis Reference(s)
The Journal of Organic Chemistry, 22, p. 1595, 1957 DOI: 10.1021/jo01363a016
General Description
Methocarbamol, 3-(o-methoxyphenoxy)-1,2-propanediol 1-carbamate (Robaxin), issaid to be more sustained in effect than mephenesin. Likelysites for metabolic attack include the secondary hydroxylgroup and the two ring positions opposite the ether functions.The dihydric parent compound, guaifenesin, is used asan expectorant.
Pharmacokinetics
Methacarbamol is a skeletal muscle relaxant with an unknown mechanism of action. Methacarbamol has been shown to block spinal polysynaptic reflexes, decrease nerve transmission in spinal and supraspinal polysynaptic pathways, and prolong the refractory period of muscle cells. Methocarbamol does not act as a local anesthetic upon injection. In animal studies, methocarbamol also prevents convulsions after electric shock.
Synthesis
Methocarbamol, 3-(2-methoxyphenoxy)-1,2-propanediol-1 carbamate (15.3.13), is synthesized by successive reaction with phosgene and then ammonia into 3- (2-methoxyphenoxy)propanediol-1,2.
Veterinary Drugs and Treatments
In dogs and cats, methocarbamol is indicated (FDA approved) “as adjunctive therapy of acute inflammatory and traumatic conditions of the skeletal muscle and to reduce muscular spasms.” In horses, intravenous use is indicated (FDA approved) “as adjunctive therapy of acute inflammatory and traumatic conditions of the skeletal muscle to reduce muscular spasms, and effect striated muscle relaxation.” (Package insert; Robaxin?V—Robins)
Metabolism
Methocarbamol is metabolized in the liver by demethylation to 3-(2-hydroxyphenoxy)-1,2-propanediol-1-carbamate or hydroxylation to 3-(4-hydroxy-2-methoxyphenoxy)-1,2-propanediol-1-carbamate. Methocarbamol and its metabolites are conjugated through glucuronidation or sulfation.
Properties of Methocarbamol
| Melting point: | 95-97°C |
| Boiling point: | 384.01°C (rough estimate) |
| Density | 1.2611 (rough estimate) |
| refractive index | 1.5080 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 13.08±0.20(Predicted) |
| form | Solid |
| color | White to Off-White |
| Merck | 14,5980 |
| InChI | InChI=1S/C11H15NO5/c1-15-9-4-2-3-5-10(9)16-6-8(13)7-17-11(12)14/h2-5,8,13H,6-7H2,1H3,(H2,12,14) |
| CAS DataBase Reference | 532-03-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Methocarbamol(532-03-6) |
| EPA Substance Registry System | Methocarbamol (532-03-6) |
Safety information for Methocarbamol
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Methocarbamol
| InChIKey | GNXFOGHNGIVQEH-UHFFFAOYSA-N |
| SMILES | C(OC(=O)N)C(O)COC1=CC=CC=C1OC |
Methocarbamol manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 METHOCARBAMOL 99%View Details
METHOCARBAMOL 99%View Details -
 Methocarbamol 99%View Details
Methocarbamol 99%View Details -
 Methocarbamol 98%View Details
Methocarbamol 98%View Details -
 Methocarbamol CAS 532-03-6View Details
Methocarbamol CAS 532-03-6View Details
532-03-6 -
 Methocarbamol >98% (HPLC) CAS 532-03-6View Details
Methocarbamol >98% (HPLC) CAS 532-03-6View Details
532-03-6 -
 Methocarbamol 98% (HPLC) CAS 532-03-6View Details
Methocarbamol 98% (HPLC) CAS 532-03-6View Details
532-03-6 -
 Methocarbamol CAS 532-03-6View Details
Methocarbamol CAS 532-03-6View Details
532-03-6 -
 Methocarbamol API UspView Details
Methocarbamol API UspView Details
532-03-6
