Methazolamide
Synonym(s):N-(4-Methyl-2-sulfamoyl-Δ2-1,3,4-thiadiazolin-5-ylidene) acetamide;5-Acetylimino-4-methyl-·2-1,3,4-thiadiazoline-2-sulfonamide;Methazolamide;N-[5-(Aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]acetamide;N-[5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]acetamide, Carbonic Anhydrase Inhibitor, Methazolamide, CAIX Inhibitor III
- CAS NO.:554-57-4
- Empirical Formula: C5H8N4O3S2
- Molecular Weight: 236.27
- MDL number: MFCD00083416
- EINECS: 209-066-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
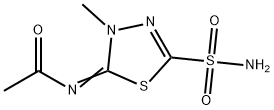
What is Methazolamide?
Absorption
Methazolamide is well absorbed from the gastrointestinal tract.
Toxicity
Electrolyte imbalance, development of an acidotic state, and central nervous system effects might be expected to occur in the case of an overdose.
Description
Methazolamide is a carbonic anhydrase inhibitor (IC50 = 130 nM). It reduces intraocular pressure and cerebrospinal fluid flow in a rat model of glaucoma. Methazolamide reduces electroshock-induced seizures in rats with an ED50 value of 19.2 mg/kg. It also inhibits production of reactive oxygen species (ROS) in a primary cortical neuron (PCN) cellular model of subarachnoid hemorrhage (SAH) and reduces cerebral edema in a mouse model of SAH. Methazolamide is larvicidal, with a larvicidal concentration (LC50) value of 724 ppm, but has no activity when administered in the diet to adult A. aegypti. Formulations containing methazolamide have been used for the treatment of glaucoma.
Chemical properties
White Solid
Originator
Neptazane ,Lederle,US,1959
The Uses of Methazolamide
Methazolamide is a carbonic anhydrase inhibitor. Methazolamide is used in the treatment of glaucoma.
The Uses of Methazolamide
CNS & respiratory stimulant
The Uses of Methazolamide
Action of this drug is similar to that of acetazolamide, and it is used for lowering intraocular pressure in treating wide-angle and secondary glaucoma, and before surgical intervention for severe wide-angle glaucoma.
Background
A carbonic anhydrase inhibitor that is used as a diuretic and in the treatment of glaucoma.
Indications
For treatment of chronic open-angle glaucoma and acute angle-closure glaucoma
What are the applications of Application
Methazolamide is a carbonic anhydrase inhibitor
Definition
ChEBI: Methazolamide is a member of thiadiazoles and a sulfonamide.
Manufacturing Process
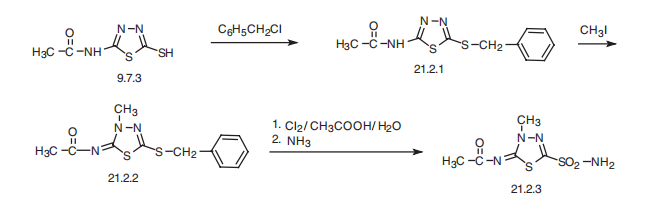
A suspension of 6 parts by weight of 5-acetylimino-4-methyl-2- benzylmercapto-δ2-1,3,4-thiadiazoline in 180 parts by volume of 33% aqueous acetic acid was chlorinated at 5°C for 30 minutes. The solid was filtered off, dried, and added portion-wise to 100 parts by volume of liquid ammonia. The ammonia was removed under a stream of dry nitrogen.
The residual solid was partially dissolved in 10 parts by volume of water, filtered, and acidified to give 5-acetylimino-4-methyl-δ2-1,3,4-thiadiazoline-2- sulfonamide. The product was purified by two recrystallizations from hot water.
Therapeutic Function
Carbonic anhydrase inhibitor
Biochem/physiol Actions
Methazolamide is a cell-permeable and potent carbonic anhydrase (CA) inhibitor that is used in the treatment of glaucoma. Methazolamide is an insulin sensitizer that reduces hepatic glucose generation in animal models.
Pharmacokinetics
Methazolamide is topical carbonic anhydrase inhibitor. Methazolamide is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to beta-blockers. Methazolamide is a sulfonamide derivative; however, it does not have any clinically significant antimicrobial properties. Although methazolamide achieves a high concentration in the cerebrospinal fluid, it is not-considered an effective anticonvulsant. Methazolamide has a weak and transient diuretic effect, therefore use results in an increase in urinary volume, with excretion of sodium, potassium and chloride.
Clinical Use
Methazolamide is a derivative of acetazolamide in which one of the active hydrogens has been replaced by a methyl group. This decreases the polarity and permits a greater penetration into the ocular fluid, where it acts as a carbonic anhydrase inhibitor, reducing intraocular pressure. Its dose for glaucoma is 50 to 100 mg two to three times a day.
Synthesis
Methazolamide, N-(4-methyl-2-sulfamoyl-1,3,4-thiadiazol-5-yliden) acetamide (21.2.3), is made by an intermediate product of acetazolamide synthesis?a 2-acetylamino-5-mercapto-1,3,4-thadiazol (9.7.3). This is benzylated with benzylchloride at the mercapto group, forming 2-acetylamino-5-benzylthio-1,3,4-thiadiazole (21.2.1). Further methylation of the product with methyl iodide leads to the formation of N-(4-methyl- 2-benzylthio-1,3,4-thiadiazol-5-yliden)acetamide (21.2.2). Oxidation and simultaneous chlorination of the resulting product with chlorine in an aqueous solution of acetic acid, and reacting the resulting chlorosulfonic derivative with ammonia gives (21.2.3).
Veterinary Drugs and Treatments
Orally administered methazolamide is used for the medical treatment of glaucoma.
Metabolism
Not Available
Properties of Methazolamide
| Melting point: | 208 °C (dec.) (lit.) |
| Boiling point: | 402.0±28.0 °C(Predicted) |
| Density | 1.6625 (rough estimate) |
| refractive index | 1.6270 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | White powder |
| pka | 7.30(at 25℃) |
| color | white to beige |
| Water Solubility | 2.835g/L(25 ºC) |
| CAS DataBase Reference | 554-57-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Methazolamide(554-57-4) |
| EPA Substance Registry System | Acetamide, N-[5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]- (554-57-4) |
Safety information for Methazolamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Methazolamide
| InChIKey | FLOSMHQXBMRNHR-QPJJXVBHSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 Methazolamide 98% CAS 554-57-4View Details
Methazolamide 98% CAS 554-57-4View Details
554-57-4 -
 Methazolamide CAS 554-57-4View Details
Methazolamide CAS 554-57-4View Details
554-57-4 -
 Methazolamide CAS 554-57-4View Details
Methazolamide CAS 554-57-4View Details
554-57-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8