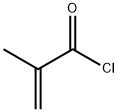
Methacrolein
Synonym(s):Methacrylaldehyde
- CAS NO.:78-85-3
- Empirical Formula: C4H6O
- Molecular Weight: 70.09
- MDL number: MFCD00006974
- EINECS: 201-150-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
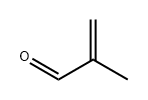
What is Methacrolein?
Chemical properties
colourless to light yellow liquid
The Uses of Methacrolein
Methacrolein was used to study the effect of parts per billion levels of limonene oxidation products and the terpene oxidation product, methacrolein on human eye blink frequency. It was also used in determination of rate constants and the products of the reactions between atomic chlorine and acrolein, methacrolein and methyl vinyl ketone.
The Uses of Methacrolein
An unsaturated aldehyde used in the manufacture of polymers and synthetic resin. Cancer studies revealed consumption and release of this volatile organic compound in human small cell lung cancer line, a potential cancer biomarker.
The Uses of Methacrolein
Methacrolein is used in the manufacturing of polymers, synthetic resins and plastics. It acts as a raw material of methylmalonic acid, thermoplastic fuel and in the preparation of 2,3-dibromo-2-methyl-propionaldehyde. It is also used to study the effect limonene oxidation products and the terpene oxidation product.
Definition
ChEBI: Methacrolein is an enal.
General Description
A colorless liquid. Flash point 35°F. Less dense than water. May be toxic by ingestion, inhalation and skin absorption. Severely irritating to skin and eyes. Used to make plastics.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
Methacrolein is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.
Hazard
Flammable, dangerous fire risk. Strong irritant.
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact with some of these materials will irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. May polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Highly flammable
Safety Profile
Poison by ingestion and sktn contact. Moderately toxic by inhalation. Severe eye and skin irritant. Mutation data reported. Dangerously flammable liquid when exposed to heat, flame, or oxidizers. Can react vigorously with oxidizing materials. To fight fire, use CO2, alcohol foam, foam, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES.
Purification Methods
Fractionally distil it under nitrogen through a short Vigreux column (p 11). Store it in sealed ampoules. (Slight polymerisation may occur.) [Beilstein 1 IV 3455.]
Properties of Methacrolein
| Melting point: | -81 °C |
| Boiling point: | 69 °C |
| Density | 0.85 g/mL at 25 °C(lit.) |
| vapor density | 2.4 (vs air) |
| vapor pressure | 121 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 5 °F |
| storage temp. | 2-8°C |
| solubility | Benzene (Slightly), Chloroform (Soluble), Methanol (Soluble) |
| form | Liquid |
| color | Pale yellow |
| Odor | at 0.01 % in dipropylene glycol. wild hyacinth foliage |
| Odor Threshold | 0.0085ppm |
| Water Solubility | 6 g/100 mL (20 ºC) |
| Sensitive | Light Sensitive |
| BRN | 1209258 |
| Exposure limits | ACGIH: TWA 1 mg/m3 OSHA: TWA 2 mg/m3 NIOSH: IDLH 50 mg/m3; Ceiling 2 mg/m3 |
| Stability: | Stable. Incompatible with strong bases, strong oxidising agents, strong reducing agents. Light and air-sensitive. Hazardous polymerisation may occur. |
| CAS DataBase Reference | 78-85-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propenal, 2-methyl-(78-85-3) |
| EPA Substance Registry System | Methacrolein (78-85-3) |
Safety information for Methacrolein
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methacrolein
| InChIKey | STNJBCKSHOAVAJ-UHFFFAOYSA-N |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

![(-)-2-[METHYLAMINO]-1-PHENYLPROPANE](https://img.chemicalbook.in/CAS/GIF/33817-09-3.gif)

You may like
-
 Methacrolein, Stabilized with hydroquinone CAS 78-85-3View Details
Methacrolein, Stabilized with hydroquinone CAS 78-85-3View Details
78-85-3 -
 Methacrolein, Stabilized with hydroquinone CAS 78-85-3View Details
Methacrolein, Stabilized with hydroquinone CAS 78-85-3View Details
78-85-3 -
 Methacrolein (stabilized with HQ) CAS 78-85-3View Details
Methacrolein (stabilized with HQ) CAS 78-85-3View Details
78-85-3 -
 Methacrolein (stabilized with HQ) 95% CAS 78-85-3View Details
Methacrolein (stabilized with HQ) 95% CAS 78-85-3View Details
78-85-3 -
 Methacrolein CAS 78-85-3View Details
Methacrolein CAS 78-85-3View Details
78-85-3 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
