Acrolein
Synonym(s):2-Propenal
- CAS NO.:107-02-8
- Empirical Formula: C3H4O
- Molecular Weight: 56.06
- MDL number: MFCD00006998
- EINECS: 203-453-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
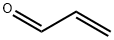
What is Acrolein?
Description
The first time that acrolein was produced as a commercial product was in the 1930s through the vapor-phase condensation of acetaldehyde and formaldehyde. Another method was developed in the 1940s, which involved the vapor-phase oxidation of propylene. In the 1960s, some advances were found in propylene oxidation process by the introduction of bismuth molybdate-based catalysis, and that became the primary method used for the commercial production of acrolein. Some bioproducts formed for this reaction are acrylic acid, carbon oxides, acetaldehyde, acetic acid, formaldehyde, and polyacrolein. In World War I, it was used as a chemical weapon (pulmonary irritant and lachrymatory agent). Commercial acrolein contains 95.5% or more of the compound, the main impurities being water (<3.0% by weight) and other carbonyl compounds (<1.5% by weight), mainly propanol and acetone. Hydroquinone is added as an inhibitor of polymerization (0.1–0.25% by weight).
Chemical properties
Acrolein is a highly flammable, clear to yellowish liquid. It has a piercing, disagreeable odor and causes tears.
Physical properties
Colorless to yellow, clear, watery liquid imparting a very sharp, acrid, pungent, or irritating odor. Odor threshold concentrations reported were 0.11 mg/kg by Guadagni et al. (1963), 0.21 ppmv by Leonardos et al. (1969), and 36 ppbv by Nagata and Takeuchi (1990). In addition, Katz and Talbert (1930) reported an experimental detection odor threshold concentration of 4.1 mg/m3 (1.8 ppmv).
The Uses of Acrolein
Acrolein is used in the synthesis of acrylic acid. manufacture of colloidal forms of metals; making plastics, perfumes; warning agent in methyl chloride refrigerant. Has been used in military poison gas mixtures. Used in organic syntheses. Aquatic herbicide.
The Uses of Acrolein
Acrolein is used as an antimicrobial agentto prevent the growth of microbes againstplugging and corrosion, to control the aquaticweed and algae, in slime control in papermanufacturing, as a tissue fixative, and inleather tanning.
Because of its widespread use it occursin the environment—in air and water. Afterformaldehyde it is the second most abundantaldehyde, constituting 5% of total aldehydesin air. Acrolein is one of the toxic gasesproduced in a wood or building fire or whenpolyethylene or other polymer substancesburn (Morikawa 1988; Morikawa and Yanai1986). Firefighters are at greater risk ofexposure to this gas.
The Uses of Acrolein
Acrolein is used in the etherification of food starch up to 0.6% and for the esterification and etherification of food starch up to 0.3% with vinyl acetate up to 7.5%.
The Uses of Acrolein
Contact herbicide and algicide; injected in water for the control of submerged and floating weeds in irrigation ditches and canals
Definition
A colorless liquid unsaturated aldehyde with a pungent odor. It can be polymerized to make acrylate resins.
Synthesis Reference(s)
Journal of the American Chemical Society, 68, p. 2487, 1946 DOI: 10.1021/ja01216a013
Air & Water Reactions
Highly flammable. A dangerous fire risk [Hawley]. Water soluble. Reacts slowly and exothermically with water to give 3-hydroxypropionaldehyde. A hazard can develop from this reaction if acrolein is stored over a layer of water.
Reactivity Profile
ACROLEIN, [INHIBITED] can react violently with oxidizing agents. Polymerizes exothermically on contact with small amounts of acids (including sulfur dioxide), alkalis, volatile amines and pyridines, salts, thiourea, oxidizing agents (air) and on exposure to light and heat. Polymerization initiated by amines and pyridines occurs after a deceptive induction period. Water solutions of mineral acids and metal ions can initiate polymerization. The inhibitor (usually hydroquinone) greatly reduces tendency to polymerize. Undergoes Diels-Alder reaction with itself to give acrolein dimer. This can become a runaway reaction at 90°C [Kirk-Othmer, 4th Ed, Vol. 1]. Mixing in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: 2-aminoethanol, ammonium hydroxide, chlorosulfonic acid, ethylenediamine, ethyleneimine [NFPA 1991].
Health Hazard
Acrolein is a highly toxic compound thatcan severely damage the eyes and respiratorysystem and burn the skin. Ingestion can causeacute gastrointestinal pain with pulmonarycongestion.
LD50 value, oral (mice): 40 mg/kg
Acrolein is a strong lachrymator and anasal irritant. Direct contact of liquid in theeyes may result in permanent injury to thecornea. Inhalation can result in severe irritationof the eyes and nose. A concentration of0.5 ppm for 12 minutes can cause intolerableeye irritation in humans. In rats, exposure toa concentration of 16 ppm acrolein in air for4 hours was lethal.
Acrolein can be absorbed through theskin; the spillage of liquid can cause severechemical burns. Skin contact may lead tochronic respiratory disease and producedelayed pulmonary edema. Subcutaneousadministration of acrolein produced degenerationof fatty liver and a general anestheticeffect.
LD50 value, subcutaneous (mice): 30 mg/kg
On the basis of the available data, aconcentration of 68 and 55 ppb may betoxic to aquatic life in fresh and salt water,respectively (U.S. EPA 1980). A concentrationas low as 21 ppb may producechronic toxicity to freshwater aquatic life.Acrolein is reported to be more toxic toaquatic organisms than are phenol, chloroandnitrophenols, aniline, o-xylene, and othertoxic compounds (Holcombe et al. 1987).Rainbow trout, spinally transected, wereexposed to an acutely toxic aqueous concentrationof acrolein to monitor their respiratory–cardiovascular responses. A steadyincrease was recorded in their cough rate.The ventilation rate, oxygen utilization, andheart rate steadily fell throughout their periodof survival.
In a study on inhalation toxicity in rats,Crane et al. (1986) observed that the exposureto 1 atm of acrolein vapors causedphysical incapacitation. The animals lost theability to walk and expired. In a study oncytotoxicity of tobacco-related aldehydes tocultured human bronchial epithelial cells,acrolein was found to be more toxic thanformaldehyde (Graftstrom et al. 1985). Bothcompounds induced DNA damage.
Certain sulfur compounds, such as dithiothreitol and dimercaptopropanol, reacted with acrolein to reduce itstoxicity (Dore et al. 1986). Such protectionagainst its toxicity was observed in isolatedrat hepatocytes.
Fire Hazard
Under fire conditions, polymerization may occur. If inside a container, violent rupture of the container may take place. When heated to decomposition, Acrolein emits highly toxic fumes. Alkalis or strong acids act as catalysts, causing a condensation reaction and liberating energy. Reaction may be very rapid and violent. Readily converted by oxygen to hazardous peroxides and acids. Unstable, avoid exposure to alkalis, strong acids, oxygen, elevated temperatures, such as fire conditions. (Polymerization inside container could cause violent rupture of container under fire conditions.)
Flammability and Explosibility
Acrolein is a highly flammable liquid (NFPA rating = 3) and its vapor can travel a considerable distance and "flash back." Acrolein vapor forms explosive mixtures with air at concentrations of 2.8 to 31% (by volume). Carbon dioxide or dry chemical extinguishers should be used for acrolein fires.
Safety Profile
Human poison by inhalation and intradermal routes. Poison experimentally by most routes. Human systemic irritant and pulmonary system effects by inhalation include: lachrymation, delayed hypersensitivity with multiple organ involvement, and respiratory system damage. Severe eye and skin irritant. Experimental reproductive effects. Human mutation data reported. Questionable carcinogen. Dangerous fire hazard when exposed to heat, flame, or oxidizers. An explosion hazard. Incompatible with amines, SO2, metal salts, oxidants, (light + heat). Violent polymerization reaction on contact with strong acid, strong base, weak acid conditions (e.g., nitrous fumes, sulfur dioxide, carbon dioxide), thiourea, or dimethylamine. When heated to decomposition it emits highly toxic fumes; can react vigorously with oxidizing materials. To fight fire, use CO2, dry chemical, or alcohol foam,
Potential Exposure
Used as pharmaceutical; slimicide; and in production of cosmetics and food supplements; as an intermediate in the production of glycerine and in the production of methionine analogs (poultry feed protein supplements). It is also used in chemical synthesis (1,3,6-hexametriol and glutaraldehyde); as a liquid fuel; antimicrobial agent, in algae and aquatic weed control; and as a slimicide in paper manufacture; making plastics, drugs, and tear gas. Also, most allyl compounds may be metabolized to allyl alcohol which is metabolized to acrolein.
Carcinogenicity
Acrolein is a reactive intermediate of the commonly used chemotherapeutic drugs cyclophosphamide and ifosphamide. Acrolein-modified DNA was found in human peripheral blood lymphocytes from cancer patients previously treated with cyclophosphamide (a chemotherapeutic), but no association was found for cyclophosphamine. Acrolein has a classification of C, possible human carcinogen, based on limited animal carcinogenicity data and paucity of human evidence for this effect.
Source
Reported in cigarette smoke (150 ppm) and gasoline exhaust (0.2 to 5.3 ppm) (quoted,
Verschueren, 1983). May be present as an impurity in 2-methoxy-3,4-dihydro-2H-pyran (Ballantyne et al., 1989a).
Acrolein was detected in diesel fuel at a concentration of 3,400 μg/g (Schauer et al., 1999).
Gas-phase tailpipe emission rates from California Phase II reformulated gasoline-powered
automobiles with and without catalytic converters were 0.06 and 3.8 mg/km, respectively (Schauer
et al., 2002).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rates of acrolein were 63 mg/kg of pine burned, 44 mg/kg of oak burned, and 56 mg/kg of
eucalyptus burned.
Environmental Fate
Biological. Microbes in site water degraded acrolein to β-hydroxypropionaldehyde
(Kobayashi and Rittman, 1982). This product also forms when acrolein is hydrated in
distilled water (Burczyk et al., 1968). When 5 and 10 mg/L of acrolein were statically
incubated in the dark at 25°C with yeast extract and settled domestic wastewater inoculum,
complete degradation was observed after 7 days (Tabak et al., 1981). Activated sludge
was capable of degrading acrolein at concentrations of 2,300 ppm but no other information
was provided (Wierzbicki and Wojcik, 1965)
Photolytic. Photolysis products include carbon monoxide, ethylene, free radicals and
a polymer (Calvert and Pitts, 1966). Anticipated products from the reaction of acrylonitrile
with ozone or hydroxyl radicals in the atmosphere are glyoxal, forma
Groundwater. The half-life for acrolein in groundwater was estimated to range from
14 days to 8 weeks (Howard et al., 1991)
Chemical/Physical. Wet oxidation of acrolein at 320°C yielded formic and acetic acids
(Randall and Knopp, 1980). May polymerize in the presence of light and explosively in
the presence of concentrated acids (Worthing and Hance, 1991) forming disacryl, a white
plastic solid (Windholz et al., 1983; Humburg et al., 1989). In distilled water, acroleinwas hydrolyzed to β-hydroxypropionaldehyde (Burczyk et al., 1968; Reinert and Rodgers,
1987; Kollig, 1993). The reported hydrolysis rate constant at pH 7 is 6.68 × 108/year
(Kollig, 1993). The estimated hydrolysis half-life in water is 22 days (Burczyk et al., 1968)
Metabolic pathway
When fish are exposed to 14C-acrolein, the metabolites are identified from the edible tissues and there is very little similarity in the metabolism of acrolein among the test species. The most notable observation is that acrolein is never detected in any tissues sampled, and glycidol, glycerol, 1,3- propanediol, and glyceric acid are the major metabolites found in catfish, crayfish, bluegill, and clams, respectively.
Storage
Work with acrolein should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and butyl rubber gloves should be worn at all times to prevent eye and skin contact. Acrolein should be used only in areas free of ignition sources. Containers of acrolein should be stored in secondary containers in areas separate from amines, oxidizers, acids, and bases.
Shipping
Acrolein, stabilized, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquids. Inhalation Hazard Zone A.
Purification Methods
Purify acrolein by fractional distillation, under nitrogen, drying with anhydrous CaSO4 and then distilling under vacuum. Blacet, Young and Roof [J Am Chem Soc 59 608 1937] distilled it under nitrogen through a 90cm column packed with glass rings. To avoid formation of diacryl, the vapour is passed through an ice-cooled condenser into a receiver cooled in an ice-salt mixture and containing 0.5g catechol. The acrolein is then distilled twice from anhydrous CuSO4 at low pressure, catechol being placed in the distilling flask and the receiver to avoid polymerization. [Alternatively, hydroquinone (1% of the final solution) can be used.] [Beilstein 1 IV 3435.]
Toxicity evaluation
The main acrolein route of exposure is through smoke. Acrolein is produced as a by-product of combustion of organic compounds, being present in a large spectrum of different smoke produced by, for example, cigarettes, petrochemical fuels (like gasoline or oil), synthetic polymers, paraffin wax, trees, plants, food, animals, vegetables fats, and building fires. Additional exposure can be linked to traffic accidents or to water treated with biocides that contain acrolein. Improperly handled hazardous waste sites can release acrolein into the nearby environment (air, water, or soil).
Incompatibilities
May form explosive mixture with air. Elevated temperatures or sunlight may cause explosive polymerization. A strong reducing agent; reacts violently with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Polymerizes exothermically on contact with small amounts of acids (including sulfur dioxide), alkalis, volatile amines and pyridines, salts, thiourea, oxidizing agents (air) and on exposure to light, and heat. Polymerization initiated by amines and pyridines occurs after a deceptive induction period. Water solutions of mineral acids and metal ions can initiate polymerization. The inhibitor (usually hydroquinone) greatly reduces tendency to polymerize. Reacts with acids, alkalis, ammonia, amines, oxygenperoxides. Shock-sensitive peroxides or acids may be formed over time. Attacks zinc and cadmium
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration. Conditions are 816 C, 0.5 second minimum for primary combustion; 1093 C, 1.0 second for secondary combustion.
Properties of Acrolein
| Melting point: | −87 °C(lit.) |
| Boiling point: | 53 °C(lit.) |
| Density | 0.839 g/mL at 25 °C(lit.) |
| vapor density | 1.94 (vs air) |
| vapor pressure | 4.05 psi ( 20 °C) |
| refractive index | n |
| Flash point: | −2 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble2 to 3 parts |
| form | Liquid |
| color | Colourless to Pale Yellow |
| Odor | Pungent, lacrimatory, intensely irritating odor detectable at 0.02 to 0.4 ppm |
| explosive limit | 31% |
| Odor Threshold | 0.0036ppm |
| Water Solubility | Soluble. 21.25 g/100 mL |
| Sensitive | Air & Light Sensitive |
| Merck | 14,128 |
| BRN | 741856 |
| Henry's Law Constant | (x 10-6 atm?m3/mol at 25 °C):
135 (Snider and Dawson, 1985) |
| Exposure limits | NIOSH REL: TWA 0.1 ppm, STEL 0.3 ppm, IDLH 2 ppm; OSHA
PEL: TWA 0.1 ppm; ACGIH TLV: TWA 0.1 ppm, STEL 0.3 ppm. |
| Stability: | Stable, but very readily polymerizes. May have ca. 0.1% hydroquinone added as stabilizer. Flammable. Incompatible with oxidizing agents, reducing agents, oxygen, a variety of other chemicals, light. Very reactive with a wide variety of chemicals. May polymerize violently, especially on contact with strong acids or bases. |
| CAS DataBase Reference | 107-02-8(CAS DataBase Reference) |
| IARC | 3 (Vol. 63, Sup 7) 1995 |
| NIST Chemistry Reference | 2-Propenal(107-02-8) |
| EPA Substance Registry System | Acrolein (107-02-8) |
Safety information for Acrolein
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H311:Acute toxicity,dermal H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acrolein
| InChIKey | HGINCPLSRVDWNT-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
