METHACHOLINE CHLORIDE
Synonym(s):(2-Acetoxypropyl)trimethylammonium chloride;Acetyl-β-methylcholine chloride;Methacholine chloride
- CAS NO.:62-51-1
- Empirical Formula: C8H18ClNO2
- Molecular Weight: 195.69
- MDL number: MFCD00011817
- EINECS: 200-537-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
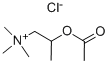
What is METHACHOLINE CHLORIDE?
Chemical properties
colourless to white crystalline powder with a slight
The Uses of METHACHOLINE CHLORIDE
antihypertensive, antianginal
The Uses of METHACHOLINE CHLORIDE
Acetyl-β-methylcholine (methacholine), an analog of acetylcholine, is use as a receptor agonist to study processes such as airway hyperresponsiveness associated with asthma.
What are the applications of Application
Acetyl-β-methylcholine chloride is a metabolically stable analog of acetylcholine
Definition
ChEBI: Methacholine chloride is a quaternary ammonium salt. It contains a methacholine.
General Description
Methacholine chloride occurs as colorless or white crystalsor as a white crystalline powder. It is odorless or has aslight odor and is very deliquescent. It is freely soluble inwater, alcohol, or chloroform, and its aqueous solution isneutral to litmus and bitter. It is hydrolyzed rapidly in alkalinesolutions. Solutions are relatively stable to heat and willkeep for at least 2 or 3 weeks when refrigerated to delaygrowth of molds.
Biochem/physiol Actions
Metabolically stable analog of acetylcholine; muscarinic agonist. Its ability to constrict airway smooth muscle is used to assess bronchial reactivity in the laboratory and clinically.
Clinical Use
Methacholine, acetyl β-methylcholine, is marketed as the racemic mixture. It is a selective muscarinic agonist with very little activity at nicotinic receptors. Although methacholine chloride is marketed as the racemic mixture, the S-(+)-enantiomer is 240-fold more potent than the R-(–)-isomer at muscarinic receptors. In addition, AChE hydrolyzes S-(+)–methacholine at approximately 54% the rate of acetylcholine, whereas the R-(–)–enantiomer is a weak inhibitor. Methacholine chloride is used via inhalation for the diagnosis of asthma. The resulting bronchospasm may be relieved with bronchodilators. Methacholine chloride is available as a powder that is reconstituted for inhalation.
Purification Methods
It forms white hygroscopic needles from Et2O and is soluble in H2O, EtOH and CHCl3. It decomposes readily in alkaline solutions and slowly in H2O. It should be handled and stored in a dry atmosphere. The bromide is less hygroscopic, and the picrate has m 129.5-131o (from EtOH). [racemate: Annis & Ely Biochem J 53 34 1953, IR of iodide: Hansen Acta Chem Scand 13 155 1959, Beilstein 4 IV 1670.]
Properties of METHACHOLINE CHLORIDE
| Melting point: | 171-173 °C(lit.) |
| Density | 1.1028 (rough estimate) |
| refractive index | 1.5790 (estimate) |
| storage temp. | -20°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | powder |
| color | White to Almost white |
| PH | 3.0~7.0 (50g/l, 25℃) |
| Water Solubility | almost transparency |
| Merck | 14,5940 |
| BRN | 4162373 |
| Stability: | Unstable - moisture sensitive. Incompatible with oxidizing agents. |
| CAS DataBase Reference | 62-51-1 |
| EPA Substance Registry System | 1-Propanaminium, 2-(acetyloxy)-N,N,N-trimethyl-, chloride (62-51-1) |
Safety information for METHACHOLINE CHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for METHACHOLINE CHLORIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![BICUCULLINE METHYLCHLORIDE (-), [METHYL-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB6479941.gif)

![N-METHYL L-QNB, [N-METHYL-3H]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB1689129.gif)
You may like
-
 Methacholine Chloride CAS 62-51-1View Details
Methacholine Chloride CAS 62-51-1View Details
62-51-1 -
 Methacholine chloride 95% CAS 62-51-1View Details
Methacholine chloride 95% CAS 62-51-1View Details
62-51-1 -
 Acetyl-β-methylcholine chloride CAS 62-51-1View Details
Acetyl-β-methylcholine chloride CAS 62-51-1View Details
62-51-1 -
 Methacholine chloride CAS 62-51-1View Details
Methacholine chloride CAS 62-51-1View Details
62-51-1 -
 Methacholine Chloride CAS 62-51-1View Details
Methacholine Chloride CAS 62-51-1View Details
62-51-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1