Mesterolone
Synonym(s):1α-Methylandrostan-17β-ol-3-one
- CAS NO.:1424-00-6
- Empirical Formula: C20H32O2
- Molecular Weight: 304.47
- MDL number: MFCD00133082
- EINECS: 215-836-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-23 19:56:12
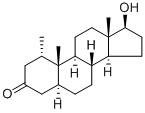
What is Mesterolone?
Description
Mesterolone (Item No. 21171) is an analytical reference standard categorized as an anabolic androgenic steroid. Formulations containing mesterolone have been used in patients with low testosterone or symptoms of aging male syndrome. Mesterolone cannot be aromatized to estrogens, unlike other androgens. This compound is the active ingredient in different pharmaceutical preparations that have been used medically but have also been widely applied in sports in order to improve athlete performance. Mesterolone is regulated as a Schedule III drug in the United States. This product is intended for research and forensic applications.
Chemical properties
White or yellowish crystalline powder.
Originator
Proviron,Schering,W. Germany,1967
The Uses of Mesterolone
Mesterolone is a synthetic androgen and a dihydrotestosterone derivative. Mesterolone is rarely used for replacement therapies due to its weak androgenic activity.
Definition
ChEBI: Mesterolone is a 3-oxo-5alpha-steroid. It is a structurally altered DHT hormone possessing the addition of a methyl group at the carbon one position. This allows the hormone to survive oral ingestion by protecting it from hepatic breakdown. This is one of the only oral anabolic steroids that is not C17-alpha alkylated (C17-aa) but instead carries the added methyl group.
What are the applications of Application
Mesterolone is a pharmacological agent that belongs to the group of androgens. It is an oral preparation and has been used in the treatment of infectious diseases, autoimmune diseases, and metabolic disorders. It has also been used as a matrix effect control in biological samples. The biological effects of it are mediated by its direct action on cells or through conversion to testosterone or dihydrotestosterone (DHT). These effects include increasing protein synthesis, promoting bone formation, lowering cholesterol levels, stimulating the production of red blood cells, and decreasing fat deposition.
Manufacturing Process
500 mg of 1α-methyl-androstan-17β-ol-3-one-17-acetate are heated under reflux for 90 minutes in a nitrogen atmosphere in 5 ml of 4% methanolic sodium hydroxide solution. The reaction mixture is then stirred into ice water,the precipitated product filtered with suction and recrystallized from isopropyl ether. 1α-Methyl-androstan-17β-ol-3-one melts at 203.5° to 205°C.
Therapeutic Function
Androgen
General Description
Mesterolone is a synthetic anabolic-androgenic steroid (AAS) and derivative of dihydrotestosterone (DHT). It is inactivated by 3α-hydroxysteroid dehydrogenase in skeleta muscules so it is considered a weak androgen. It is not a substrate for aromatase so it is not converted into estrogen. Mesterolone demonstrated to have minimal effect on sperm counts and levels of FSH or LH. Experiments of mesterolone serving as a potential treatment of depression are still undergoing.
Side Effects
The main side effects are acne vulgaris, male pattern baldness, prostate enlargement, increased risk for prostate cancer, and increased risk for developing breast cancer.
Properties of Mesterolone
| Melting point: | 208 °C |
| Boiling point: | 420.3±45.0 °C(Predicted) |
| alpha | D20 +17.6° (c = 0.875 in CHCl3) |
| Density | 1.156 g/cm3 |
| refractive index | 17.6 ° (C=0.9, CHCl3) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, sparingly soluble in acetone, in ethyl acetate and in methanol |
| form | neat |
| pka | 15.09±0.70(Predicted) |
| Water Solubility | 3mg/L at 20℃ |
| Merck | 5916 |
| InChI | InChI=1/C20H32O2/c1-12-10-14(21)11-13-4-5-15-16-6-7-18(22)19(16,2)9-8-17(15)20(12,13)3/h12-13,15-18,22H,4-11H2,1-3H3/t12-,13-,15-,16-,17-,18-,19-,20-/s3 |
| CAS DataBase Reference | 1424-00-6(CAS DataBase Reference) |
Safety information for Mesterolone
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H351:Carcinogenicity H362:Reproductive toxicity, effects on or via lactation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Mesterolone
| InChIKey | UXYRZJKIQKRJCF-TZPFWLJSSA-N |
| SMILES | [C@@]12([H])CC[C@@]3([H])CC(=O)C[C@H](C)[C@]3(C)[C@@]1([H])CC[C@]1(C)[C@H](CC[C@@]21[H])O |&1:0,4,10,12,14,18,20,23,r| |
Mesterolone manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 Mesterolone 99%View Details
Mesterolone 99%View Details -
 1424-00-6 98%View Details
1424-00-6 98%View Details
1424-00-6 -
 Mesterolone 98%View Details
Mesterolone 98%View Details
1424-00-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8