MERCURIC NITRATE
- CAS NO.:10045-94-0
- Empirical Formula: HgN2O6
- Molecular Weight: 324.6
- MDL number: MFCD00011038
- EINECS: 233-152-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
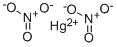
What is MERCURIC NITRATE?
Description
Mercuric Nitrate is a white to yellowish crystalline solid with a nitric acid-like odor. Normally exists as hemihydrate or dihydrate. Molecular weight= 324.61;Boiling point=(decomposes); Freezing/Melting point=7079℃. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 0, Reactivity 0. Soluble in water.
Chemical properties
white to yellow crystalline powder with nitric acid odour
Chemical properties
Mercuric nitrate is a white to yellowish crystalline solid with an odor like nitric acid. Normally exists as the hemihydrate or the dihydrate
The Uses of MERCURIC NITRATE
Nitration of aromatic organic compounds, felt manufacture, mercury fulminate manufacturing.
General Description
A white crystalline solid. Toxic by inhalation, ingestion and/or skin contact. Prolonged exposure to fire or heat may result in an explosion. Produces toxic oxides of nitrogen when heated to decomposition. Used to make other chemicals and in medicine.
Air & Water Reactions
Deliquescent. Soluble in a small amount of water. With much water or on boiling with water, an insoluble basic salt is formed.
Reactivity Profile
MERCURIC NITRATE is noncombustible, but, as an oxidizing agent, will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Light sensitive. Mixtures with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. Acetylene forms a sensitive acetylide when passed into an aqueous solution of MERCURIC NITRATE [Mellor 4:933. 1946-47]. Should not be mixed with alcohols as explosive mercury fulminates may be formed [Bahme 1961. p. 9]. Is violently reduced by hypophosphoric acid [Mellor 4:993. 1946-47]. Reacts with phosphine to give a yellow precipitate that explodes when heated or subjected to shock [Mellor 4:993. 1946-47].
Hazard
Dangerous fire risk in contact with organic materials. Very toxic.
Health Hazard
Acute systemic poisoning may be fatal within a few minutes; death by uremic poisoning is usually delayed 5-12 days. Acute poisoning has resulted from inhaling dust concentrations of 1.2-8.5 mg/m 3 of air; symptoms inc lude tightness and pain in chest, coughing, and difficulty in breathing. Ingestion causes necrosis, pain, vomiting, and severe purging. Contact with eyes causes ulceration of conjunctiva and cornea. Contact with skin causes irritation and po ssible dermatitis; systemic poisoning can occur by absorption through skin.
Safety Profile
Poison by ingestion, skin contact, intraperitoneal, and subcutaneous routes. A powerful oxidizer. Probably an eye, skin, and mucous membrane irritant. Reacts with acetylene to form the explosive mercury acetylide whch is sensitive to heat, friction, or contact with sulfuric acid. Reaction with ethanol forms the explosive mercury fulrmnate. Reaction with isobutene forms an unstable explosive product. Forms explosive mixtures with phosphine (heatand impact-sensitive), potassium cyanide (heat-sensitive), and sulfur. Violent reaction with phosphinic acid, hypophosphoric acid, unsaturated hydrocarbons, aromatics. Vigorous reaction with petroleum hydrocarbons. When heated to decomposition it emits very toxic fumes of Hg and NOx. See also MERCURY COMPOUNDS, INORGANIC; and NITRATES.
Potential Exposure
Mercuric nitrate is used in making other chemicals; in felt manufacture and in making mercury fulminate
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Antidotes and special procedures for medical personnel: The drug NAP (n-acetyl penicillamine) has been used to treat mercury poisoning, with mixed success. Note to physician: For severe poisoning BAL [British AntiLewisite, dimercaprol, dithiopropanol (C3H8OS2)] has been used to treat toxic symptoms of certain heavy metals poisoning including mercury. Although BAL is reported to have a large margin of safety, caution must be exercised, because toxic effects may be caused by excessive dosage. Most can be prevented by premedication with 1-ephedrine sulfate (CAS: 134-72-5).
Storage
Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with this chemical you should be trained on its proper handling and storage. Mercuric nitrate must be stored to avoid contact with organic materials; acetylene, ethanol, phosphine, sulfur, and hypophosphoric acid, since violent reactions occur. See also “Incompatibilities.” Do not store on wooden floors.
Shipping
UN1625 Mercuric nitrate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
A strong oxidizer. Reacts violently with combustibles, petroleum hydrocarbons; reducing agents; aldehydes, ammonia, ketones, phosphorus. Reacts with acetylene, alcohol, phosphine, and sulfur to form shocksensitive compounds. Aqueous solution attacks most metals. Vigorous and dangerous reaction with petroleum hydrocarbons. Incompatible with organic materials; acetylene, ethanol, phosphine, sulfur, hypophosphoric acid. Inorganic mercury compounds are incompatible with acetylene, ammonia, chlorine dioxide; azides, calcium (amalgam formation), sodium carbide; lithium, rubidium, copper. Decomposes in heat or on exposure to light, producing toxic fumes (mercury, nitrogen oxides)
Properties of MERCURIC NITRATE
| Melting point: | 79°C |
| Boiling point: | decomposes [CRC10] |
| Density | 1.025 g/mL at 25 °C |
| solubility | soluble in H2O; insoluble in ethanol |
| form | colorless hygroscopic crystals |
| color | colorless hygroscopic crystals, crystalline |
| Water Solubility | soluble H2O; insoluble EtOH [CRC10] |
| Stability: | Stable. Incompatible with strong reducing agents, combustible materials, most common metals. |
| CAS DataBase Reference | 10045-94-0(CAS DataBase Reference) |
| EPA Substance Registry System | Mercuric nitrate (10045-94-0) |
Safety information for MERCURIC NITRATE
Computed Descriptors for MERCURIC NITRATE
MERCURIC NITRATE manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


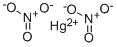


You may like
-
 Mercury Nitrate, For Ayurvedic Medicine, Grade: A GradeView Details
Mercury Nitrate, For Ayurvedic Medicine, Grade: A GradeView Details
10045-94-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
