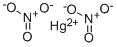CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Mercuric nitrate appears as a white crystalline solid. Toxic by inhalation, ingestion and/or skin contact. Prolonged exposure to fire or heat may result in an explosion. Produces toxic oxides of nitrogen when heated to decomposition. Used to make other chemicals and in medicine. |
|---|---|
| Color/Form | White or slightly yellow crystal powder |
| Odor | Sharp odor |
| Melting Point | 79 °C |
| Solubility | VERY SOL IN COLD WATER; SOL IN ACETONE, NITRIC ACID, AMMONIA; INSOL IN ALCOHOL /MERCURIC NITRATE HEMIHYDRATE/ |
| Density | 4.3 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Stability/Shelf Life | Decomposed by heat |
| Corrosivity | Solution will corrode most metals. |
| Other Experimental Properties | Mercuric nitrate dissolves in water, then forms a cloudy acid solution. The reaction is not hazardous. |
| Chemical Classes | Metals -> Mercury Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 324.60 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 325.946279 g/mol |
| Monoisotopic Mass | 325.946279 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Mercuric nitrate appears as a white crystalline solid. Toxic by inhalation, ingestion and/or skin contact. Prolonged exposure to fire or heat may result in an explosion. Produces toxic oxides of nitrogen when heated to decomposition. Used to make other chemicals and in medicine.