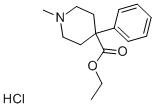
Mepivacaine hydrochloride
Synonym(s):1-Methyl-2′,6′-pipecoloxylidine hydrochloride;1-Methyl-2,6-pipecoloxylidine hydrochloride;Carbocaine hydrochloride;Mepivacaine hydrochloride;N-(2,6-Dimethylphenyl)-1-methyl-2-piperidinecarboxamide hydrochloride
- CAS NO.:1722-62-9
- Empirical Formula: C15H22N2O.ClH
- Molecular Weight: 282.81
- MDL number: MFCD00144738
- EINECS: 217-023-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
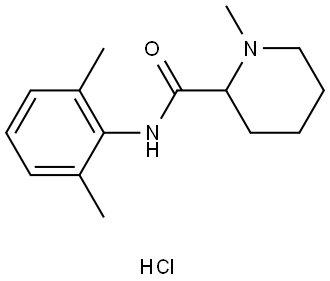
What is Mepivacaine hydrochloride?
Chemical properties
White Solid
The Uses of Mepivacaine hydrochloride
Mepivacaine hydrochloride may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by chromatography.
The Uses of Mepivacaine hydrochloride
An anti-inflammatory and analgesic
Definition
ChEBI: The hydrochloride salt of mepivacaine. It is used as a local anaesthetic.
brand name
Arestocaine Hydrochloride (Solvay Pharmaceuticals); Carbocaine (Hospira); Isocaine Hydrochloride(Novocol); Polocaine (AstraZeneca); Polocaine (Dentsply); Scandonest (Deproco).
Biological Functions
Mepivacaine hydrochloride (Carbocaine) is longer acting than lidocaine and has a more rapid onset of action (3–5 minutes). Topical application is not effective. It has been widely used in obstetrics, but its use has declined recently because of the early transient neurobehavioral effects it produces. Adverse reactions associated with mepivacaine are generally similar to those produced by other local anesthetics. It can be used with epinephrine or levonordefrin (dental use only).
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. Mepivacaine hydrochloride is a local anesthetic that is related chemically and pharmacologically to the amide-type local anesthetics. It is often administered via local infiltration, peripheral nerve block, etc.
Biochem/physiol Actions
Local anesthetic. Reversibly blocks transient Na+ inward current, as well as the steady-state K+ outward current. Blocks tandem pore (TASK) and Kv1.5, potassium channels in model systems.
Safety Profile
skin irritant. Questionable carcinogen with
Mechanism of action
Mepivacaine hydrochloride acts by binding to specific membrane sodium ion channels in the neuronal cell membranes, thereby inhibiting sodium influx. This leads to a blockage of nerve impulse conduction and results in a loss of sensation.
Properties of Mepivacaine hydrochloride
| Melting point: | 255-257°C (dec.) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| InChI | InChI=1S/C15H22N2O.ClH/c1-11-7-6-8-12(2)14(11)16-15(18)13-9-4-5-10-17(13)3;/h6-8,13H,4-5,9-10H2,1-3H3,(H,16,18);1H |
| CAS DataBase Reference | 1722-62-9(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Piperidinecarboxamide, N-(2,6-dimethylphenyl)-1-methyl-, monohydrochloride (1722-62-9) |
Safety information for Mepivacaine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Mepivacaine hydrochloride
| InChIKey | RETIMRUQNCDCQB-UHFFFAOYSA-N |
| SMILES | C1(=C(C)C=CC=C1C)NC(=O)C1CCCCN1C.Cl |
Mepivacaine hydrochloride manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 1722-62-9 95-99 %View Details
1722-62-9 95-99 %View Details
1722-62-9 -
 Mepivacaine Hydrochloride CAS 1722-62-9View Details
Mepivacaine Hydrochloride CAS 1722-62-9View Details
1722-62-9 -
 Mepivacaine HCl 95% CAS 1722-62-9View Details
Mepivacaine HCl 95% CAS 1722-62-9View Details
1722-62-9 -
 Mepivacaine hydrochloride CAS 1722-62-9View Details
Mepivacaine hydrochloride CAS 1722-62-9View Details
1722-62-9 -
 Mepivacaine hydrochloride CAS 1722-62-9View Details
Mepivacaine hydrochloride CAS 1722-62-9View Details
1722-62-9 -
 Mepivacaine hydrochloride CAS 1722-62-9View Details
Mepivacaine hydrochloride CAS 1722-62-9View Details
1722-62-9 -
 Mepivacaine Hydrochloride PowderView Details
Mepivacaine Hydrochloride PowderView Details
1722-62-9 -
 Mepivacaine Hydrochloride (1722-62-9)View Details
Mepivacaine Hydrochloride (1722-62-9)View Details
1722-62-9
