Donepezil Hydrochloride
Synonym(s):(±)-2-[(1-Benzyl-4-piperidyl)methyl]-5,6-dimethoxy-1-indanone hydrochloride;1H-Inden-1-one, 2,3-dihydro-5,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-, hydrochloride;2,3-Dihydro-5,6-dimethoxy-2-{[1-(phenylmethyl)-4-piperidinyl]methyl}-1H-inden-1-one;Donepezil hydrochloride;E2020
- CAS NO.:120011-70-3
- Empirical Formula: C24H30ClNO3
- Molecular Weight: 415.95
- MDL number: MFCD00881312
- EINECS: 620-543-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-02 16:58:49
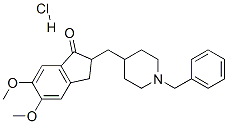
What is Donepezil Hydrochloride?
Chemical properties
Donepezil hydrochloride (E2020) alongside with galantamine and rivastigmine is a piperidine-based drug developed specifically for Alzheimer’s disease (AD) by Eisai Pharmaceuticals (Aricept). Donepezil hydrochloride is commonly referred to in the pharmacological literature as E2020. It has an empirical formula of C24H29NO3HCl and a molecular weight of 415.96. Donepezil hydrochloride is a white crystalline powder and is freely soluble in chloroform, soluble in water and in glacial acetic acid, slightly soluble in ethanol and in acetonitrile, and practically insoluble in ethyl acetate and in n-hexane.
Originator
Eisai (Japan)
The Uses of Donepezil Hydrochloride
Donepezil hydrochloride is an inhibitor of acetylcholinesterase with a labelled nootropic. It has been used in dose-response experiment to study its efficacy in ameliorating nicotine withdrawal-induced deficits in contextual conditioning in mice. It has also been intraperitoneally injected into rats to study its ability to improve social behavioral deficits, repetitive behavior and hyperactivity. Labeled Donepezil, intended for use as an internal standard for the quantification of Donepezilby GC- or LC-mass spectrometry.
What are the applications of Application
Donepezil hydrochloride is a noncompetitive acetylcholinesterase (AChE) inhibitor
Definition
ChEBI: Donepezil hydrochloride is a centrally acting reversible acetyl cholinesterase inhibitor. Its main therapeutic use is in the treatment of Alzheimer's disease where it is used to increase cortical acetylcholine.
What are the applications of Application
Donepezil hydrochloride is a reversible,non-competitiveChEl with highly selective central action,approved for thetreatment of mild, moderate and advanced AD. Donepezil is unrelated to other ChEIs and has greaterselectivity for cerebral acetylcholinesterase (AChE) than forbutirylcholinesterase (BuChE).
Preparation
Donepezil Hydrochloride is being synthesized from alkylidene or arylidene-2-indanone formed by Aldol condensation chemistry as key intermediates (Sugimoto and co-workers)) followed by catalytic reduction affording donepezil hydrochloride with an overall yield of 27%.
Benzyl chloride (1.92 g, 0.0156 mol), potassium carbonate (2.28 g, 0.0165 mol) and PEG-200 (80 mg, 2% by weight) were added to a clear biphasic solution of 2-[(4-piperidyl)methyl]-5,6-dimethoxyindan-1-one 6 (4 g, 0.0138 mol) in 9 : 1 EtOAc–H2O (40 ml). The reaction mixture was heated to 50—55 °C and monitored on TLC. After completion of reaction (14 h), the solid was filtered and the filtrate was washed with water (4 ml). The EtOAc layer was separated, added water (8 ml) and acidified with conc. HCl till a pH 2. EtOAc layer was discarded and the acidic aqueous layer was extracted with CH2Cl2 (2*16 ml). The CH2Cl2 layer was separated, dried over anhydrous Na2SO4, filtered and evaporated under vacuum to yield the crude salt as a residue. The residue was dissolved in methanol (20 ml) followed by the addition of isopropyl ether (40 ml) and stirred for a further 1 h. The precipitated solid was filtered and dried under vacuum to yield pure donepezil hydrochloride.
A New Commercially Viable Synthetic Route for Donepezil Hydrochloride
brand name
Aricept (Eisai Medical Research).
Therapeutic Function
Anti-Alzheimer's disease
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Donepezil Hydrochloride is known as a selective, reversible inhibitor of acetylcholinesterase enzyme. It exhibits therapeutic effect by raising the acetylcholine concentrations and improving the cholinergic function. It can be used in the treatment of mild to moderate dementia of the Alzheimer′s type.
Biological Activity
donepezil hydrochloride (donepezil hcl) is a novel, potent and selective inhibitor of acetylcholinesterase (ache), an enzyme possibly involved in cognitive dysfunction of patients suffering alzheimer’s disease (ad), with the half maximal inhibition concentration ic50 value of 6.7 nm [1].donepezil hcl is a piperidine-class ache inhibitor containing an n-benzylpiperdine and an indanone moiety, which confers it a longer and more selective action against ache as compared to buche (ic50: 7.4 μm) [1].donepezil hcl has been approved by fda for the treatment of ad, in which it has improved cognitive function of mild to severe moderate ad patients and exhibited excellent tolerability without hepatotoxicity [1].
Biochem/physiol Actions
Donepezil hydrochloride is a piperidine containing an organic compound and a non-competitive inhibitor. It is useful in treating Alzheimer′s disease, in which dementia is a prominent symptom. Donepezil is shown to induce cognitive ability and overall body function. It has a half-life of 70 hours.
Pharmacology
Donepezil hydrochloride is a second-generation highly selective acetylcholinesterase inhibitor developed by the Japanese company Eisai Pharmaceutical Company. It was first marketed in the United States in 1997 for the treatment of Alzheimer's disease. Donepezil is the second drug approved by the FDA for the treatment of Alzheimer's disease. It is a highly selective, long-acting, reversible acetylcholinesterase inhibitor with a piperidine group. Compared with tacrine, donepezil hydrochloride is more selective for neuronal acetylcholinease, has better pharmacodynamics, pharmacokinetics and safety indicators, and is well tolerated. It is an alternative drug to tacrine.
Donepezil hydrochloride is a cholinesterase inhibitor. After taking it, patients mainly increase the content of acetylcholine in the synapses that are directly involved in nerve transmission by inhibiting the activity of acetylcholine lipase. Donepezil hydrochloride can Effectively improve patients 'brain function and improve patients' cognitive ability. Currently, donepezil hydrochloride is a commonly used drug in the treatment of mild to moderate senile dementia. Donepezil hydrochloride tablets are suitable for the treatment of memory degeneration of dementia caused by various reasons. Donepezil hydrochloride has the effect of nourishing brain neurons, repairing damaged brain nerves, and can rapidly improve patients' cognitive function and memory.
Clinical Use
Treatment of dementia in mild to moderate Alzheimer’s disease
Side Effects
Donepezil was generally well tolerated, although more adverse effects were reported in the 10 mg/day group than assessed as mild to moderate and transient, and were typi-cally diarrhoea,nausea,arthralgia,leg cramps,anorexia and headache. Donepezil (5-10 mg/day) had no effecton V-ADAS-cog but had significant effect on two measuresof executive function: EXIT25 and TMT-B. These subjectswere younger than most VaD patients and most (75%) hadno clinically significant memory dysfunction. The positive effect of donepezil on executive function, however, indicatessome cholinergic deficit in executive dysfunction.
Metabolism
Donepezil hydrochloride is both excreted in the urine
intact and metabolised by the cytochrome P450
system to multiple metabolites, not all of which have
been identified. Following administration of a single
5 mg dose of [14C]-labelled donepezil hydrochloride,
plasma radioactivity, expressed as a percentage of the
administered dose, was present primarily as intact
donepezil hydrochloride (30%), 6-O-desmethyl donepezil
(11% - only metabolite that exhibits activity similar to
donepezil hydrochloride), donepezil-cis-N-oxide (9%),
5-O-desmethyl donepezil (7%) and the glucuronide
conjugate of 5-O-desmethyl donepezil (3%).
Approximately 57% of the total administered
radioactivity was recovered from the urine, and 14.5% was
recovered from the faeces, suggesting biotransformation
and urinary excretion as the primary routes of
elimination. There is no evidence to suggest enterohepatic
storage
Room Temperature
Properties of Donepezil Hydrochloride
| Melting point: | 220-222°C |
| storage temp. | room temp |
| Water Solubility | Soluble in water |
| solubility | insoluble in EtOH; insoluble in DMSO; ≥10.4 mg/mL in H2O |
| form | powder |
| color | white to off-white |
| Merck | 14,3419 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 120011-70-3(CAS DataBase Reference) |
Safety information for Donepezil Hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Donepezil Hydrochloride
| InChIKey | XWAIAVWHZJNZQQ-UHFFFAOYSA-N |
Donepezil Hydrochloride manufacturer
SETV ASRV LLP
New Products
Ethyl 3,3- diethoxypropionate 7-Bromo-2-chloroquinoxaline N-Benzyl-3-hydroxypiperidine 2-(4-bromophenyl)-2-methylpropanoic acid Benzylacetoacetate Radiator Flux Zinc Chloride Solution (All Grades) Phenylazomalononitrile 2-Fluoro-6-iodobenzoic acid 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 2,3-Difluoro-6-methoxybenzyl Chloride 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Ethyl2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate Acetyl-meldrum's acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II BromideRelated products of tetrahydrofuran

You may like
-
 Donepezil hydrochloride 98%View Details
Donepezil hydrochloride 98%View Details -
 DONEPEZIL HCL 98%View Details
DONEPEZIL HCL 98%View Details -
 120011-70-3 DONEPEZIL HCL IP 95-99 %View Details
120011-70-3 DONEPEZIL HCL IP 95-99 %View Details
120011-70-3 -
 Donepezil hydrochloride, ≥98% CAS 120011-70-3View Details
Donepezil hydrochloride, ≥98% CAS 120011-70-3View Details
120011-70-3 -
 Donepezil hydrochloride 98%View Details
Donepezil hydrochloride 98%View Details -
 Donepezil hydrochloride 99%View Details
Donepezil hydrochloride 99%View Details -
 Donepezil HCl 99% (HPLC) CAS 120011-70-3View Details
Donepezil HCl 99% (HPLC) CAS 120011-70-3View Details
120011-70-3 -
 Donepezil HCl 95.00% CAS 120011-70-3View Details
Donepezil HCl 95.00% CAS 120011-70-3View Details
120011-70-3
