D-Biotin
Synonym(s):Biotin;Vitamin B7;Vitamin H;D-(+)-Biotin;D(+)-Biotin
- CAS NO.:58-85-5
- Empirical Formula: C10H16N2O3S
- Molecular Weight: 244.31
- MDL number: MFCD00005541
- EINECS: 200-399-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
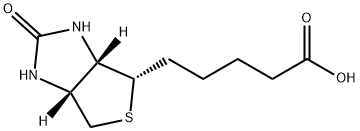
What is D-Biotin?
Absorption
Systemic - approximately 50%
Toxicity
Prolonged skin contact may cause irritation.
Sources
Biotin is mainly derived from offal, eggs, fish, meat, seeds, nuts and certain vegetables (e.g. sweet potatoes), which are the foods with the highest biotin content. The specific amounts of biotin in different foods are shown in the table below: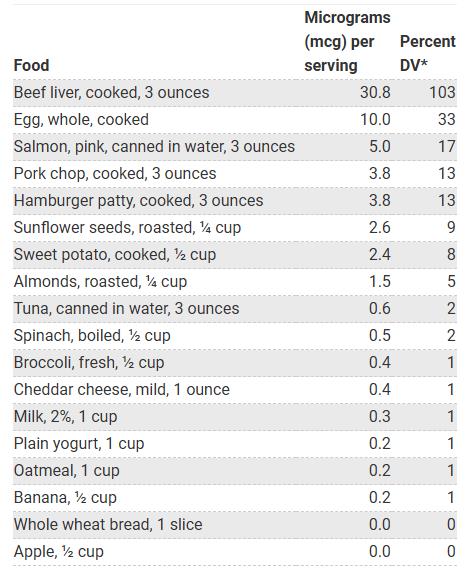
Description
Biotin, a water-soluble B-complex vitamin, is integral to cell growth, fatty acid production, and metabolism. It plays a crucial role in the Kreb cycle, aiding in energy release from food and facilitating metabolic conversions and carbon dioxide transfer. Known for maintaining blood sugar levels and promoting hair and nail strength, biotin is prevalent in cosmetic and health products. Biotin deficiency, while rare, can lead to dry skin, seborrheic dermatitis, fungal infections, hair loss, neurological symptoms, and in infants, "cradle cap." In adults, it may result in anemia, depression, hair loss, high blood sugar, muscle pain, nausea, loss of appetite, and inflamed mucous membranes. Treatment typically involves biotin supplementation.
Chemical properties
White powder
Originator
Biotin,Solgar
The Uses of D-Biotin
Biotin is a B vitamin, an essential nutrient that occurs naturally in certain foods and is also used as a dietary supplement. This water-soluble vitamin is a cofactor for five carboxylases (propionyl-CoA carboxylase, pyruvate carboxylase, methylcrotonyl-CoA carboxylase [MCC], acetyl-CoA carboxylase 1, and acetyl-CoA carboxylase 2). Biotin also plays a key role in histone modification, gene regulation (by altering the activity of transcription factors), and cell signalling.
The Uses of D-Biotin
vitamin B complex
The Uses of D-Biotin
Growth factor present in minute amounts in every living cell. Plays an indispensable role in numerous naturally occurring carboxylation reactions. Occurs mainly bound to proteins or polypeptides. The richest sources are liver, kidney, pancreas, yeast, and milk. The biotin content of cancerous tumors is higher than that of normal tissue.
Indications
For nutritional supplementation, also for treating dietary shortage or imbalance.
What are the applications of Application
D-(+)-Biotin is an important ubiquitous metabolism coenzyme
Background
A water-soluble, enzyme co-factor present in minute amounts in every living cell. It occurs mainly bound to proteins or polypeptides and is abundant in liver, kidney, pancreas, yeast, and milk.
Definition
ChEBI: An organic heterobicyclic compound that consists of 2-oxohexahydro-1H-thieno[3,4-d]imidazole having a valeric acid substituent attached to the tetrahydrothiophene ring. The parent of the class of biotins.
Manufacturing Process
4-Carbomethoxy-2-(4,5-dihydrothiophen-3(2H)-one)valeric acid methyl ester
was prepared from 4,5-dihydrothiophene as it was described in Baker et al., J.
Org. Chem., 12, 167 (1947).
A solution of 60.0 g (0.182 mole) this ester in 550 ml absolute ethanol was
treated with 91.6 g (1.45 moles) of ammonium formate. The reaction mixture
refluxed for 5.0 hours. Then it was cooled, concentrated, and partitionated in
a separatory funnel between 200 ml dichloromethane and 150 ml water. The
aqueous phase was extracted three times with 50 ml portions of
dichloromethane. The organic extracts were collected, dried over anhydrous
sodium sulfate, and evaporated. 50 g (0.182 mole, 100%) 3-amino-4-
carbomethoxy-2,5-dihydro-2-thiophenevaleric acid methyl ester was obtained
as a colorless oil.
To a solution of 27.3 g (1 mole) of 3-amino-4-carbomethoxy-2,5-dihydro-2-
thiophenevaleric acid methyl ester in 250 ml dry methanol was added 4.0 g
(0.1 mole) of sodium hydroxide pellets. The reaction mixture was refluxed 4.0
hrs, cooled and concentrated to a volume of 50 ml. The residue was taken up
in 80 ml dichloromethane and transfered to a separatory funnel. After the
addition of 150 ml of 10% by weight aqueous sodium bicarbonate solution,
the aqueous layer was extracted twice with 50 ml portions of
dichloromethane. The organic phases were combined, dried over anhydrous
sodium sulfate, and evaporated to yield 6.4 g (0.0234 mole) of recovered
starting material. The aqueous phase was adjusted to pH 1 with 6 N
hydrochloric acid and extracted three times with 75 ml portions of
dichloromethane. The organic phases were pooled, dried over anhydrous
sodium sulfate, and evaporated to yield 18.3 g (0.071 mole, 71%) of 3-
amino-4-carbomethoxy-2,5-dihydro-2-thiophenevaleric acid as a tan solid,
upon trituration with pet. ether.
The recovered starting material, 6.4 g (0.0234 mole) was dissolved in 70 ml
dry methanol and treated with 1.0 g (0.025 mole) sodium hydroxide. The
mixture was refluxed 5.0 hrs, cooled concentrated, and taken up in 80 ml
dichloromethane. The organic phase was treated in a separatory funnel with
100 ml of 10% by weight aqueous sodium bicarbonate solution. The aqueous
phase was extracted twice with 40 ml portions of dichloromethane. The
aqueous phase was acidified to pH 1 with 6 N hydrochloric acid and extracted
two times with 50 ml portions of dichloromethane. The organic phases were
cooled, dried over anhydrous sodium sulfate, and evaporated to dryness to
afford an additional 5.3 g (0.021 mole, 21%) of 3-amino-4-carbomethoxy-
2,5-dihydro-2-thiophenevaleric acid; m.p. 98°-102°C.
brand name
Bioepiderm (Sterling Winthrop).
Therapeutic Function
Vitamin
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Biotin is a water-soluble vitamin, essential for amino acids and carbohydrates metabolism. It is involved in de novo synthesis of purine nucleotides and plays a role in gene expression and DNA replication.
Biochem/physiol Actions
Biotin is a vital cofactor for carboxylase enzymes in several metabolic?pathways. It also functions as a coenzyme in the metabolism of fatty acids, isoleucine and valine. Biotin assists the transfer of carbon dioxide and also sustains a steady blood sugar level. Biotin is implicated in gluconeogenesis and citric acid cycle. It?is involved in?keratin?synthesis and hence?is used as?a supplement?for skin, hair and nail growth. Biotin is required for cell growth, production of fatty acids and the metabolism of fats and amino acids.
Safety Profile
An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx and SOx.
Purification Methods
D-(+)-Biotin crystallises from hot water in fine long needles with a solubility of 22 mg/100mL at 25o. Its solubility in 95% EtOH is 80 mg/100 mL at 25o. Its isoelectric point is at pH 3.5. Store solid and solutions under sterile conditions because it is susceptible to mould growth. [Confalone J Am Chem Soc 97 5936 1975, Wolf et al. J Am Chem Soc 67 2100 1945, Synthesis: Ohuri & Emoto Tetrahedron Lett 2765 1975, Harris et al. J Am Chem Soc 66 1756 1944.] The (+)-methyl ester has m 166-167o (from MeOH/Et2O), [] D 22 +57o (c 1, CHCl3) [du Vigneaud et al. J Biol Chem 140 643, 763 1941]; the (+)-S-oxide has m 200-203o, [] D 20 +130o (c 1.2, 0.1N NaOH) [Melville J Biol Chem 208 495 1954]; the SS-dioxide has m 274-275o(dec, 268-270o), and the SS-dioxide methyl ester has m 239-241o (from MeOH/Et2O) [Hofmann et al. J Biol Chem 141 207, 213 1941]. [Beilstein 27 III/IV 7979.]
Properties of D-Biotin
| Melting point: | 231-233 °C(lit.) |
| Boiling point: | 573.6±35.0 °C(Predicted) |
| alpha | 89 º (c=1, 0.1N NaOH) |
| Density | 1.2693 (rough estimate) |
| refractive index | 90.5 ° (C=2, 0.1mol/L NaOH) |
| storage temp. | -20°C |
| solubility | H2O: 0.2 mg/mL Solubility increases with addition of 1 N NaOH. |
| form | powder |
| pka | 4.74±0.10(Predicted) |
| color | White crystalline powder or fine long needles |
| PH | 4.5 (0.1g/l, H2O) |
| optical activity | [α]20/D +91±2°, c = 1% in 0.1 M NaOH |
| Water Solubility | Soluble in hot water, dimethyl sulfoxide, alcohol and benzene. |
| Sensitive | Light Sensitive |
| Merck | 14,1231 |
| BRN | 86838 |
| Stability: | Stable, but light sensitive. Incompatible with strong oxidizing agents, strong bases, strong acids, formaldehyde, chloramine-T, nitrous acid. |
| CAS DataBase Reference | 58-85-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Biotin(58-85-5) |
| EPA Substance Registry System | Biotin (58-85-5) |
Safety information for D-Biotin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for D-Biotin
| InChIKey | YBJHBAHKTGYVGT-ZKWXMUAHSA-N |
D-Biotin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![1H-Thieno[3,4-d]iMidazole-4-pentanaMide, hexahydro-N-[2-(4-hydroxyphenyl)ethyl]-2-oxo-, (3aS,4S,6aR)-](https://img.chemicalbook.in/CAS/GIF/41994-02-9.gif)


![[3aS,4S,6aR,(+)]-Hexahydro-2-oxo-1H-thieno[3,4-d]imidazole-4-pentanoic acid 5-oxide](https://img.chemicalbook.in/CAS/GIF/3376-83-8.gif)
You may like
-
 D-Biotin 99%View Details
D-Biotin 99%View Details -
 D-Biotin for tissue culture CAS 58-85-5View Details
D-Biotin for tissue culture CAS 58-85-5View Details
58-85-5 -
 D-Biotin CAS 58-85-5View Details
D-Biotin CAS 58-85-5View Details
58-85-5 -
 D-Biotin 98% CAS 58-85-5View Details
D-Biotin 98% CAS 58-85-5View Details
58-85-5 -
 D Biotin Powder, Grade Standard: Food Grade, 1 kg Pack, BagView Details
D Biotin Powder, Grade Standard: Food Grade, 1 kg Pack, BagView Details
58-85-5 -
 Biotin Vitamin B7, Corrurated Box, 5 KGView Details
Biotin Vitamin B7, Corrurated Box, 5 KGView Details
58-85-5 -
 D-BIOTIN 98% For Biochemistry, Packaging Type: DrumView Details
D-BIOTIN 98% For Biochemistry, Packaging Type: DrumView Details
58-85-5 -
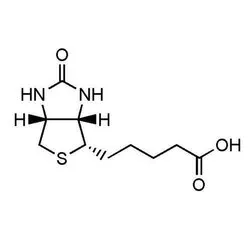 D-Biotin (CAS Number: 58-85-5)View Details
D-Biotin (CAS Number: 58-85-5)View Details
58-85-5
