Tocopheryl acetate
Synonym(s):Vitamin E acetate;all-rac-α-Tocopheryl acetate;O-Acetyl-α-tocopherol;DL -α-Tocopherol acetate;DL -α-Tocopheryl acetate
- CAS NO.:7695-91-2
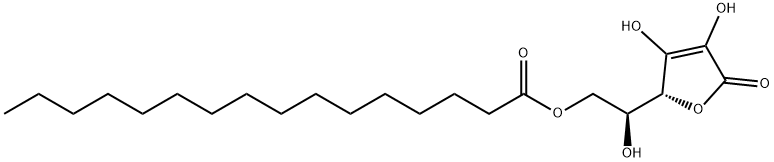
- Empirical Formula: C31H52O3
- Molecular Weight: 472.74
- MDL number: MFCD00072042
- EINECS: 231-710-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
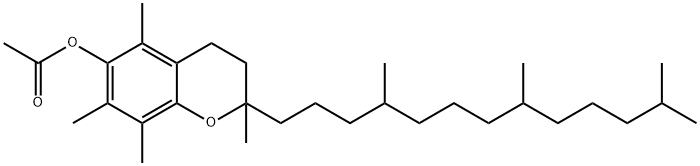
What is Tocopheryl acetate?
Absorption
When vitamin E is ingested, intestinal absorption plays a principal role in limiting its bioavailability . It is known that vitamin E is a fat-soluble vitamin that follows the intestinal absorption, hepatic metabolism, and cellular uptake processes of other lipophilic molecules and lipids . The intestinal absorption of vitamin E consequently requires the presence of lipid-rich foods .
In particular, stable alpha-tocopherol acetate undergoes hydrolysis by bile acid-dependant lipase in the pancreas or by an intestinal mucosal esterase . Subsequent absorption in the duodenum occurs by way of transfer from emulsion fat globules to water-soluble multi- and unilamellar vesicles and mixed micelles made up of phospholipids and bile acids . As the uptake of vitamin E into enterocytes is less efficient compared to other types of lipids, this could potentially explain the relatively low bioavailability of vitamin E . Alpha-tocopherol acetate itself is embedded in matrices where its hydrolysis and its uptake by intestinal cells are markedly less efficient than in mixed micelles . Subsequently, the intestinal cellular uptake of vitamin E from mixed micelles follows in principle two different pathways across enterocytes: (a) via passive diffusion, and (b) via receptor-mediated transport with various cellular transports like scavenger receptor class B type 1, Niemann-Pick C1-like protein, ATP-binding cassette (ABC) transporters ABCG5/ABCG8, or ABCA1, among others .
Vitamin E absorption from the intestinal lumen is dependent upon biliary and pancreatic secretions, micelle formation, uptake into enterocytes, and chylomicron secretion . Defects at any step can lead to impaired absorption. . Chylomicron secretion is required for vitamin E absorption and is a particularly important factor for efficient absorption. All of the various vitamin E forms show similar apparent efficiencies of intestinal absorption and subsequent secretion in chylomicrons . During chylomicron catabolism, some vitamin E is distributed to all the circulating lipoproteins .
Chylomicron remnants, containing newly absorbed vitamin E, are then taken up by the liver . Vitamin E is secreted from the liver in very low density lipoproteins (VLDLs). Plasma vitamin E concentrations depend upon the secretion of vitamin E from the liver, and only one form of vitamin E, alpha-tocopherol, is ever preferentially resecreted by the liver . The liver is consequently responsible for discriminating between tocopherols and the preferential plasma enrichment with alpha-tocopherol . In the liver, the alpha-tocopherol transfer protein (alpha-TTP) likely is in charge of the discriminatory function, where RRR- or d-alpha-tocopherol possesses the greatest affinity for alpha-TTP .
It is nevertheless believed that only a small amount of administered vitamin E is actually absorbed. In two individuals with gastric carcinoma and lymphatic leukemia, the respective fractional absorption in the lymphatics was only 21 and 29 percent of label from meals containing alpha-tocopherol and alpha-tocopheryl acetate, respectively .
Additionally, after feeding three separate single doses of 125 mg, 250 mg, and 500 mg to a group of healthy males, the observed plasma peak concentrations (ng/mL) were 1822 +/- 48.24, 1931.00 +/- 92.54, and 2188 +/- 147.61, respectively .
Toxicity
Tocopherols are considered as non-toxic but if very high doses (approximately >2 g/kg/day) are administered, there are reports of hemorrhagic activity . Reproductive and developmental toxicity tests are negative . These negative results were also observed in the analysis of mutagenicity and carcinogenicity . The majority of these tests were animal feeding studies .
Chemical properties
Light yellow to yellow clear liquid
The Uses of Tocopheryl acetate
All-rac-alpha-tocopheryl acetate for peak identification may be used as an analytical reference standard for the determination of the analyte in pharmaceutical formulations by liquid chromatography.
The Uses of Tocopheryl acetate
anti-Altzheimer therapeutic
The Uses of Tocopheryl acetate
vitamin E acetate (tocopherol acetate) is an anti-oxidant with skinmoisturizing activity. given its free-radical scavenging properties, it is useful in uV protective products. Vitamin e acetate is commonly used to replace vitamin e because it is more stable and is converted to vitamin e by the body.
The Uses of Tocopheryl acetate
Vitamin E (α-tocopherol) occurs naturally in most vegetable oils. The highest concentrations are found in com, soybean oils, sunflower seed, wheat germ, rapeseed, alfalfa, and lettuce. It is claimed to have age-retardant properties. May produce erythemamultiforme-like eruptions. It is used in a number of cosmetic products; creams are used for scars, striae, and bums; in pharmaeeutical ereams and deodorants; as an antioxidant in foods.
Background
Alpha-tocopherol is the primary form of vitamin E that is preferentially used by the human body to meet appropriate dietary requirements. In particular, the RRR-alpha-tocopherol (or sometimes called the d-alpha-tocopherol stereoisomer) stereoisomer is considered the natural formation of alpha-tocopherol and generally exhibits the greatest bioavailability out of all of the alpha-tocopherol stereoisomers. Moreover, RRR-alpha-tocopherol acetate is a relatively stabilized form of vitamin E that is most commonly used as a food additive when needed .
Alpha-tocopherol acetate is subsequently most commonly indicated for dietary supplementation in individuals who may demonstrate a genuine deficiency in vitamin E. Vitamin E itself is naturally found in various foods, added to others, or used in commercially available products as a dietary supplement. The recommended dietary allowances (RDAs) for vitamin E alpha-tocopherol are: males = 4 mg (6 IU) females = 4 mg (6 IU) in ages 0-6 months, males = 5 mg (7.5 IU) females = 5 mg (7.5 IU) in ages 7-12 months, males = 6 mg (9 IU) females = 6 mg (9 IU) in ages 1-3 years, males = 7 mg (10.4 IU) females = 7 mg (10.4 IU) in ages 4-8 years, males = 11 mg (16.4 IU) females = 11 mg (16.4 IU) in ages 9-13 years, males = 15 mg (22.4 IU) females = 15 mg (22.4 IU) pregnancy = 15 mg (22.4 IU) lactation = 19 mg (28.4 IU) in ages 14+ years . Most individuals obtain adequate vitamin E intake from their diets; genuine vitamin E deficiency is considered to be rare.
Nevertheless, vitamin E is known to be a fat-soluble antioxidant that has the capability to neutralize endogenous free radicals. This biologic action of vitamin E consequently continues to generate ongoing interest and study in whether or not its antioxidant abilities may be used to help assist in preventing or treating a number of different conditions like cardiovascular disease, ocular conditions, diabetes, cancer and more. At the moment however, there exists a lack of formal data and evidence to support any such additional indications for vitamin E use.
Indications
The primary health-related use for which alpha-tocopherol acetate is formally indicated is as a dietary supplement for patients who demonstrate a genuine vitamin E deficiency. At the same time, vitamin E deficiency is generally quite rare but may occur in premature babies of very low birth weight (< 1500 grams), individuals with fat-malabsorption disorders (as fat is required for the digestive tract to absorb vitamin E), or individuals with abetalipoproteinemia - a rare, inherited disorder that causes poor absorption of dietary fat - who require extremely large doses of supplemental vitamin E daily (around 100 mg/kg or 5-10 g/day) . In all such cases, alpha-tocopherol is largely the preferred form of vitamin E to be administered.
Elsewhere, vitamin E's chemical profile as a fat-soluble antioxidant that is capable of neutralizing free radicals in the body continues to generate ongoing interest and study regarding how and whether or not the vitamin can help prevent or delay various chronic diseases associated with free radicals or other potential biological effects that vitamin E possesses like cardiovascular diseases, diabetes, ocular conditions, immune illnesses, cancer, and more . None of these ongoing studies have yet to elucidate any formally significant evidence, however .
What are the applications of Application
DL-α-Tocopherol acetate is a form of tocopherol preferentially absorbed by humans
Definition
ChEBI: DL-alpha-Tocopherol acetate is a tocol.
General Description
DL-α-Tocopherol acetate is a stable ester form of vitamin E, widely used in the formulation of cosmetics for the prevention or correction of skin damage.
Hazard
A reproductive hazard.
Flammability and Explosibility
Non flammable
Contact allergens
Tocopherol and tocopheryl acetate are used mainly as antioxidants. Tocopheryl acetate, an ester of tocopherol (vitamin E), can induce allergic contact dermatitis.
Biochem/physiol Actions
Tocopherols are lipid soluble anti-oxidants that protect cell membranes from oxidative damage. α-Tocopherol is the form of tocopherol preferentially absorbed by Homosapiens. DL-α-Tocopherol acetate can inhibit oxidation of linoleate.
Pharmacokinetics
Of the eight separate variants of vitamin E, alpha-tocopherol is the predominant form of vitamin E in human and animal tissues, and it has the highest bioavailability . This is because the liver preferentially resecretes only alpha-tocopherol by way of the hepatic alpha-tocopherol transfer protein (alpha-TTP); the liver metabolizes and excretes all the other vitamin E variants, which is why blood and cellular concentrations of other forms of vitamin E other than alpha-tocopherol are ultimately lower .
Furthermore, the term alpha-tocopherol generally refers to a group of eight possible stereoisomers which is often called all-rac-tocopherol for being a racemic mixture of all eight stereoisomers . Of the eight stereoisomers, the RRR-alpha-tocopherol - or sometimes referred to as the d-alpha-tocopherol - stereoisomer is the naturally occurring form of alpha-tocopherol that is perhaps best recognized by the alpha-TTP and has been reported to demonstrate approximately twice the systemic availability of all-rac-tocopherol .
As a result, often times (but certainly not always) the discussion of vitamin E - at least within the context of using the vitamin for health-related indications - is generally in reference to the use of RRR- or d-alpha-tocopherol.
Metabolism
Primary hepatic metabolism of alpha-tocopherol begins in the endoplasmic reticulum with CYP4F2/CYP3A4 dependent ω-hydroxylation of the aliphatic side-chain, which forms the 13’-hydroxychromanol (13’-OH) metabolite . Next, peroxisome ω-oxidation results in 13’-carboxychromanol (13’-COOH) . Following these two steps are five consecutive β-oxidation reactions which serve to shorten the alpha-tocopherol metabolite side-chains . The first of these β-oxidations occurs still in the peroxisome environment, generating carboxydimethyldecylhydroxychromanol (CDMDHC, 11’-COOH) . Then, in the mitochondrion, the second β-oxidation step forms the carboxymethyloctylhydroxychromanol (CDMOHC, 9’-COOH) metabolite . Since both CDMDHC and CDMOHC possess a side-chain length of between 13 to 9 carbon units, they are considered long-chain metabolites. The hydrophobicity of these long-chain metabolites means they are not excreted in the urine but have been found in human microsomes, serum, and feces . The next two β-oxidation reactions, still within the mitochondrion environment, produce two intermediate chain metabolites: carboxymethylhexylhydroxychromanol (CDMHHC, 7’-COOH), followed by carboxymethylbutylhydroxychromanol (CMBHC, 5’-COOH) . Both of these intermediate chain metabolites are found in human plasma, feces, and urine . Finally, the last mitochrondrion β-oxidation generates the catabolic end product of alpha-tocopherol metabolism: carboxyethyl-hydroxychromans (CEHC, 3'-COOH), which is considered a short-chain metabolite . CEHC has been observed in human plasma, serum, urine, and feces .
Purification Methods
It is a viscous liquid which is purified by distillation under high vacuum under N2 or Ar and stored in sealed ampoules in the dark. It is considerably more stable to light and air than the parent unacetylated vitamin. It is insoluble in H2O but freely soluble in organic solvents. All eight stereoisomers have been synthesised. The commercially pure d-tocopheryl acetate (2R,4'R,8'R) has b 180-200o/0.7mm and [] D 20 +3.9o (c 5, EtOH); see above. [Cohen et al. Helv Chim Acta 64 1158 1981, Beilstein 17/4 V 169.]
Properties of Tocopheryl acetate
| Melting point: | -28°C |
| Boiling point: | 224 °C0.3 mm Hg(lit.) |
| Density | 0.96 g/mL at 20 °C (lit.) |
| vapor density | 16.3 (vs air) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in acetone, in anhydrous ethanol and in fatty oils. |
| form | neat |
| form | Viscous Liquid |
| Specific Gravity | 0.962 (20/4℃) |
| color | Clear yellow viscous liquid |
| Odor | Odorless |
| Water Solubility | Immiscible with water. |
| Sensitive | Air & Light Sensitive |
| Merck | 14,9495 |
| BRN | 97512 |
| CAS DataBase Reference | 7695-91-2(CAS DataBase Reference) |
| NIST Chemistry Reference | (+)-«ALPHA»-tocopherol acetate(7695-91-2) |
| EPA Substance Registry System | 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, acetate (7695-91-2) |
Safety information for Tocopheryl acetate
Computed Descriptors for Tocopheryl acetate
| InChIKey | ZAKOWWREFLAJOT-CEFNRUSXSA-N |
New Products
3-pyridine carboxaldehyde 7-bromoquinoxalin-2(1H)-one 2-Bromo-6-flurobenzaldehyde 8-Bromoisoquinoline 3-Pyridineacetonitrile, α-amino-, hydrochloride (1:1) Ethyl 3-Phenoxypropionate 4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE TosMIC 2-Iodo-5-methoxybenzoic acid Xanomeline Suzetrigine Remibrutinib Dimethyl 4-aminothiophene-2,3-dicarboxylate hydrochloride 1H-Indole-1-carboxylic acid, 5-hydroxy-7-methyl-, 1,1-dimethylethyl ester N N N'Trimethyl ethylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N' DimethylEthylenediamine Ethyl Methanesulfonate N Ethylmethylamine Radiator Flux Zinc Chloride Solution (All Grades) Calcium Alphaketoglutarate* Fmoc-L-Tyr(tBu)-OHRelated products of tetrahydrofuran
You may like
-
 DL-α-Tocopherol Acetate CAS 7695-91-2View Details
DL-α-Tocopherol Acetate CAS 7695-91-2View Details
7695-91-2 -
 DL-a-Tocopherol Acetate (Vitamin E Acetate) for tissue culture CAS 7695-91-2View Details
DL-a-Tocopherol Acetate (Vitamin E Acetate) for tissue culture CAS 7695-91-2View Details
7695-91-2 -
 Vitamin E acetate 98% (GC) CAS 7695-91-2View Details
Vitamin E acetate 98% (GC) CAS 7695-91-2View Details
7695-91-2 -
 DL-α-Tocopherol acetate CAS 7695-91-2View Details
DL-α-Tocopherol acetate CAS 7695-91-2View Details
7695-91-2 -
 DL-a-TOCOPHEROL ACETATE For Biochemistry CAS 7695-91-2View Details
DL-a-TOCOPHEROL ACETATE For Biochemistry CAS 7695-91-2View Details
7695-91-2 -
 Alpha Tocopheryl Acetate CAS 7695-91-2View Details
Alpha Tocopheryl Acetate CAS 7695-91-2View Details
7695-91-2 -
 DL-alpha-Tocopherol acetate CAS 7695-91-2View Details
DL-alpha-Tocopherol acetate CAS 7695-91-2View Details
7695-91-2 -
 DL-α-Tocopherol acetate CAS 7695-91-2View Details
DL-α-Tocopherol acetate CAS 7695-91-2View Details
7695-91-2
