5-Methoxytryptamine
Synonym(s):5-Methoxytryptamine;5-Methoxytryptamine hydrochloride;2-(5-Methoxyindol-3-yl)ethylamine;3-(2-Aminoethyl)-5-methoxyindole;5-MOT
- CAS NO.:608-07-1
- Empirical Formula: C11H14N2O
- Molecular Weight: 190.24
- MDL number: MFCD00005662
- EINECS: 210-153-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-05 14:33:18
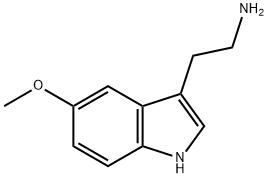
What is 5-Methoxytryptamine ?
Description
5-Methoxytryptamine (5-MT), also known as mexamine, is a tryptamine derivative closely related to the neurotransmitters serotonin and melatonin. 5-MT has been shown to occur naturally in the body in low levels.It is biosynthesized via the deacetylation of melatonin in the pineal gland.
The protective effect of 5-methoxytryptamine (a metabolite of melatonin) in human keratinocytes against ultraviolet B (UVB) radiation was studied.
Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes.
Chemical properties
White to tan crystalline powder
The Uses of 5-Methoxytryptamine
5-Methoxytryptamine (Mexamine, Methoxytryptamine) is a tryptamine derivative that is closely related to the neurotransmitter Melatonin (M215000) and Serotonin (S274980). It acts as a full agonist at the 5-HT1, 5-HT2, 5-HT4, 5-HT6, and 5-HT7 receptors but has no affinity for the 5-HT3 receptor.
What are the applications of Application
5-Methoxytryptamine is a nonselective serotonin receptor agonist
Definition
ChEBI: 5-methoxytryptamine is a member of the class of tryptamines that is the methyl ether derivative of serotonin. It has a role as a serotonergic agonist, a human metabolite, a mouse metabolite, a 5-hydroxytryptamine 2A receptor agonist, a 5-hydroxytryptamine 2C receptor agonist, a 5-hydroxytryptamine 2B receptor agonist, an antioxidant, a radiation protective agent, a neuroprotective agent and a cardioprotective agent. It is a member of tryptamines, an aromatic ether and a primary amino compound. It derives from a serotonin. It is a conjugate base of a 5-methoxytryptamine(1+).
What are the applications of Application
5-Methoxytryptamine was used as an agonist in the study of pharmacological profile of the 5-hydroxytryptamine 1 receptor.
Reactant for preparation of:
Carboline disaccharide domain of shishijimicin A
Melatonin analogs for the reduction of intraocular pressure
5-HT4 receptor ligands
inhibitors of sortase A and isocitrate lyase
Therapeutic agents for treatment of ischemia/reperfusion (I/R) injury
Aurora and epidermal growth factor receptor kinase inhibitors
Agents for the treatment of human papillomavirus infection
Manzamine analogues for the control of neuroinflammation and cerebral infections
Inhibitors of pro-inflammatory cytokines
Tacrine-melatonin hybrids as multifunctional agents for alzheimer′s disease
General Description
The protective effect of 5-methoxytryptamine (a metabolite of melatonin) in human keratinocytes against ultraviolet B (UVB) radiation was studied.
Biochem/physiol Actions
Nonselective serotonin receptor agonist that lacks affinity for the 5-HT3 receptor.
Synthesis
5-Methoxytryptamine (358) was synthesized from 3-(2-iodoethyl)-5-methoxyindole (176) by reaction with 1-methyl-benzylamine (MeCN, 24 h, RT) and subsequent catalytic debenzylation of 44 (H2, Pd/C, EtOH, 24 h, RT, 4 bar). The resulting 5-methoxytryptamine (358 was then reacted with 4-bromobenzoylchloride (THF, NEt3, RT, ON) and the resulting tryptamide 359 was reduced with aluminum hydride to N-(4-bromobenzyl)-5-methoxytryptamine (19) (LiAlH4, AlCl3, Et2O, 5 h, RT), which was isolated as its hydrogen oxalate salt.
Clinical claims and research
The effects of the 5-HT receptor agonist, 5-methoxytryptamine, on plasma glucose levels were investigated in rats. 5-Methoxytryptamine induced a significant hyperglycemia above the dosage of 1 mg/kg. 5-Methoxytryptamine-induced hyperglycemia was antagonized by pretreatment with the 5-HT1 and 5-HT2 receptor antagonist, methysergide, or the 5-HT2A receptor antagonist, ketanserin, whereas the 5-HT3 and 5-HT4 receptor antagonist, tropisetron, and the 5-HT4 receptor antagonist, SDZ 205-557 (2-methoxy-4-amino-5-chloro-benzoic acid 2-(diethylamino) ethyl ester), showed no effect. In addition, the peripheral 5-HT2 receptor antagonist, xylamidine, reduced 5-methoxytryptamine-induced hyperglycemia.
Hyperglycemia induced by the 5-HT receptor agonist, 5-methoxytryptamine, in rats: involvement of the peripheral 5-HT2A receptor.
Properties of 5-Methoxytryptamine
| Melting point: | 121-123 °C (lit.) |
| Boiling point: | 325.75°C (rough estimate) |
| Density | 1.0815 (rough estimate) |
| refractive index | 1.5700 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | crystalline |
| pka | 16.91±0.30(Predicted) |
| color | white |
| Merck | 13,6032 |
| BRN | 145587 |
| InChI | InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 |
| CAS DataBase Reference | 608-07-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 5-Methoxytryptamine(608-07-1) |
Safety information for 5-Methoxytryptamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for 5-Methoxytryptamine
| InChIKey | JTEJPPKMYBDEMY-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=C(OC)C=C2)C(CCN)=C1 |
5-Methoxytryptamine manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


![ACETYL-5'-METHOXYTRYPTAMINE, N-[METHOXY-14C],N-ACETYL-5'-METHOXYTRYPTAMINE, [METHOXY-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB5209354.gif)


You may like
-
 5-Methoxytryptamine, 97% CAS 608-07-1View Details
5-Methoxytryptamine, 97% CAS 608-07-1View Details
608-07-1 -
 5-Methoxytryptamine CAS 608-07-1View Details
5-Methoxytryptamine CAS 608-07-1View Details
608-07-1 -
 Melatonin Related Compound A 99% (HPLC) CAS 608-07-1View Details
Melatonin Related Compound A 99% (HPLC) CAS 608-07-1View Details
608-07-1 -
 Melatonin Related Compound A CAS 608-07-1View Details
Melatonin Related Compound A CAS 608-07-1View Details
608-07-1 -
 Melatonin Related Compound A CAS 608-07-1View Details
Melatonin Related Compound A CAS 608-07-1View Details
608-07-1 -
 5-Methoxytryptamine CAS 608-07-1View Details
5-Methoxytryptamine CAS 608-07-1View Details
608-07-1 -
 MELATONINE EP IMPURITY C 608-07-1 90 % AboveView Details
MELATONINE EP IMPURITY C 608-07-1 90 % AboveView Details
608-07-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
