Medrogestone
- CAS NO.:977-79-7
- Empirical Formula: C23H32O2
- Molecular Weight: 340.5
- MDL number: MFCD00271888
- EINECS: 213-555-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:44
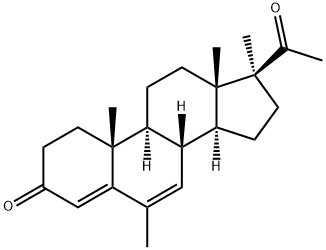
What is Medrogestone?
Absorption
When administered, medrogestone presents a very rapid gastrointestinal absorption with a bioavailability of 100%. The maximum serum concentration of medrogestone is 10-15 ng/ml.
Toxicity
There were reports of increased urinary flow rates and total micturition volumes as well as sexual dysfunction and hyperglycemia.
Originator
Colpro,Ayerst,Italy,1970
The Uses of Medrogestone
Progestin.
Indications
Medrogestone is indicated as adjunct to treat endometial shedding in menopausal women, to treat secondary amenorrhea, to induce menses and to treat dysfunctional uterine bleeding in adult and adolescent women.
Background
Medrogestone (INN), also known as 6,17α-dimethyl-6-dehydroprogesterone, is a progestational agent derived from 17-methylprogesterone. It was conceived as an alternative for an orally effective contraceptive option. It was developed by Ayerst, approved in Canada in 1969 and its current status is cancelled post-marketing. It was never approved by the FDA.
Definition
ChEBI: Medrogestone is a corticosteroid hormone.
Manufacturing Process
The manufacturing process as described in US Patent 3,170,936 uses the readily available methyl 3β-hydroxy-17α-methyl-δ5-etienate (I), described by Plattner in Helv. Chim. Acta, vol. 31, p 603 (1948), as the starting material. The etienic acid ester (I) may also be called 17α-methyl-17βcarbomethoxyandrost-5-ene-3β-ol.
3β,5α,6β-Trihydroxy-17α-Methyl-17β-Carbomethoxyandrostane (II): 5 g of
17α-Methyl-17β-carbomethoxyandrost-5-ene-3β-ol (I) is dissolved in formic
acid (50 ml) and heated on the steam bath for 10 minutes. The solution is
cooled to room temperature and a crystalline solid precipitates. This is stirred,
30% hydrogen peroxide (5 ml) is added, and the reaction mixture is left at
room temperature for 2 hours. The clear solution is poured into water 1300
ml) and the solid which precipitates is filtered.
It is dissolved in hot methanol and heated on the steam bath with 10%
methanolic potassium hydroxide solution (15.8 ml) for 10 minutes. Then more
potassium hydroxide solution (2 ml) is added, the solution is cooled and on
dilution with water a solid (II), MP 245° to 255°C, is obtained. A second crop
is obtained from the mother liquors. Several recrystallizations from acetone
yield an analytical sample, MP 262° to 265°C, [α]D24 is -2.1°.
3β-Acetoxy-5α-Hydroxy-17α-Methyl-17β-Carbomethoxyandrostane-6-one
(IIIb): 3β,5α,6βp-Trihydroxy-17α-methyl-17β-carbomethoxyandrostane (II, 5.2
g) is dissolved in methanol (105 ml) to which ether (105 ml) and water (84
ml) are added. Then N-bromosuccinimide (5.2 g) is added with stirring and
the clear solution is left in the refrigerator for 3 hours. The ether is removed
under reduced pressure at room temperature and a crystalline solid (IIIa)
separates, MP 268° to 272°C.
The above substance is dissolved in pyridine (15 ml) and acetic anhydride
(7.5 ml), and heated on the steam bath for ? hour. The product (IIIb)
crystallizes from aqueous ethanol in leaflets, MP 237° to 239°C. An analytical
sample has MP 241° to 243°C.
3β,5α,6β-Trihydroxy-6α,17α-Dimethyl-17β-Carbomethoxyandrostane (IV): 3βAcetoxy-5α-hydroxy-17α-methyl-17β-carbomethoxyandrostan-6-one (III,
1.004 g) is dissolved in dry benzene (25 ml) and methyl magnesium bromide
solution in ether (3 M, 10 ml) is added. The reaction mixture is diluted with
dry tetrahydrofuran (25 ml) and allowed to stand at room temperature for 20
hours, Excess Grignard reagent is quenched by adding a saturated solution of
ammonium chloride. The organic layer is separated and the aqueous layer is
extracted with ethyl acetate.
After washing the combined extracts with ammonium chloride solution and
water and working up in the usual way a white solid (IV) is obtained which
after one recrystallization from aqueous methanol has MP 242° to 243°C. The
infrared spectrum of this compound indicates the presence of a carbomethoxy
group (1,730 cm-1) and disappearance of the 6-keto group together with the
presence of an ester group (1,727 cm-1). This substance is used without
further purification for the next step.
3β,5α,6β-Trihydroxy-6α,17α-Dimethylpregnan-20-one (V): Crude 3β,5α,6βtrihydroxy-6α,17α-dimethyl-17β-carbomethoxyandrostane (IV, 773 mg) is
dissolved in dry benzene (25 ml) and tetrahydrofuran (freshly distilled over
lithium aluminum hydride, 25 ml). To the stirred solution under dry N2 there is
added methyl magnesium bromide solution in ether (3 M, 10 ml) over a
period of 10 minutes. Then the ether and tetrahydrofuran are almost all
distilled and the resulting solution is refluxed for 3 hours (solid precipitates
during the reaction). The reaction mixture is cooled and worked up in the
same way as in the previous experiment leaving a white solid (V) with an
infrared spectrum which indicates the presence of a 20-ketone group (1,690
cm-1), a sample of which is recrystallized to MP 238° to 240°C.
Analysis confirmed the empirical formula C23H38O4H2O: Required: C, 69.60%;
H, 10.17%. Found: C, 69.90%; H, 10.15%.
Alternatively, 25.0 g of either 3β,5α-dihydroxy-17α-methy-17βcarbomethoxyandrostan-6-one (IIIa) or 25.0 g of its 3β-acetate (IIIb), are
dissolved in dry tetrahydrofuran (1,250 ml, freshly distilled over lithium
aluminum hydride) and dry benzene (2,000 ml) is added. Methyl magnesium
bromide in ether solution (3 M, 750 ml) is added to the stirred solution and
the resulting mixture is stirred at room temperature for 16 hours. An
additional quantity of methyl magnesium bromide solution in ether (2 M, 375
ml) is added, and 1,250 ml of the solvent mixture are distilled off. The
resulting mixture is refluxed for 5 hours and worked up as described above,
yielding compound (V) as a colorless oil.
5α,6β-Dihydroxy-6α,17α-Dimethylpregnane-3,20-dione (VI): Crude 3β,5α,6βtrihydroxy-6α,17α-Dimethylpregnan-20-one (V, 650 mg) is dissolved in
acetone (freshly distilled over potassium permanganate, 150 ml) and cooled in
an ice-water bath with stirring. Then excess chromic acid solution (8 N) is
added and stirring is continued at room temperature for 4 minutes. The
reaction mixture is poured into water and extracted with ethyl acetate. The
combined extracts are washed with dilute sodium bicarbonate solution and
water and then dried over magnesium sulfate. Removal of the solvent leaves a
white solid (VI). This crude product is used for the next step. Its IR spectrum
shows a strong band at 1,705 cm-1. A sample is recrystallized to MP 243° to
245°C (dec.).
6,17α-Dimethyl-4,6-Pregnadiene-3,20-dione (VII): 5α,6β-Dihydroxy-6α,17αdimethylpregnane-3,20-dione (VI, 553 mg) is dissolved in absolute ethanol
(60 ml) and two drops of concentrated hydrochloric acid are added. This
solution is heated on a steam bath for 45 minutes, cooled, diluted with water
and extracted with ether. The combined extracts are washed with dilute
sodium bicarbonate solution and water and subsequently dried over
magnesium sulfate. After the solvent has been removed a syrup remains and
the UV spectrum of this substance indicates the presence of a δ4,6-
ketone.Elution of this material over alumina (Woelm, Grade III, 25 g) with 1:1
hexane-benzene gives a crystalline substance, MP 138° to 141°C which, after
one recrystallization from ether, has an infrared spectrum identical to that of
an authentic sample of 6,17α-dimethyl-4,6-pregnadiene-3,20-dione (VII).
Therapeutic Function
Progestin
Pharmacokinetics
Medrogestone was created as a more potent and orally active option of progesterone. In pre-clinical trials, medrogestone was proven to have four times more progestational activity than progesterone with a similar duration effect than the one found for 17-hydroxyprogesterone. Medrogestone was also able to maintain pregnancy and prevented ovulation in ovariectomized rats. Administration of medrogestone, alone or with premarin, prevented pregnancy, as well as it suppressed ovarian weight increase by nearly 100% of the tested individuals. Medrogestone does not produce any androgenic effect but it presented a marked anti-androgenic effect. It did not present an oestrogenic effect, nor changes in organ weight or histological appearance in adrenal glands or thymus and it does not present any anti-inflammatory effects.
Metabolism
The non-protein bound fraction of medrogestoneis available for metabolism. The main route in the metabolism of medrogestone is hydroxylation.
Properties of Medrogestone
| Melting point: | 144-146° |
| Boiling point: | 416.28°C (rough estimate) |
| alpha | D23 +79° (c = 1 in chloroform) |
| Density | 1.0555 (rough estimate) |
| refractive index | 1.4480 (estimate) |
| Water Solubility | 1.82mg/L(25 ºC) |
| CAS DataBase Reference | 977-79-7(CAS DataBase Reference) |
Safety information for Medrogestone
Computed Descriptors for Medrogestone
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
