Mannitol
Synonym(s):D -Mannitol;D-Mannitol;Mannite
- CAS NO.:87-78-5
- Empirical Formula: C6H14O6
- Molecular Weight: 182.17
- MDL number: MFCD00064287
- EINECS: 201-770-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
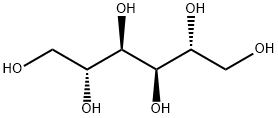
What is Mannitol?
Description
Mannitol can be used to lower intraocular pressure and in the postoperational period in ophthalomological procedures as well as during brain edema.
Chemical properties
D-mannitol (D-mannohexan-1.2.3.4.5.6-hexaol) is a constituent of several plants
including the Manna ash, several edible plants, and seaweed. Parts of the latter
contain up to 10 % mannitol by weight. The solubility in water is approximately 230 g/L at room temperature and it increases with increasing temperature. Mannitol
is stable under the common processing conditions of foods.
Mannitol is approximately 50 % as sweet as sucrose and non-cariogenic.
In the European Union, maltitol is approved as E 421 for a large number of food
applications. In the United States, mannitol produced by hydrogenation of glucose
or fructose solutions or by fermentation by Zygosaccharomyces rouxii or Lactobacillus
intermedius is approved for several food applications. It is also approved
in many other countries.
Mannitol application
Mannitol is an osmotic diuretic agent and a weak renal vasodilator.
The Uses of Mannitol
Mannitol is a polyol (polyhydric alcohol) produced from hydrogena- tion from fructose that functions as a sweetener, humectant, and bulking agent. it has low hygroscopicity and poor oil solvency. it has 1.6 kcal/g. it is approximately 22% soluble in water and is approximately 72% as sweet as sugar, exhibiting a cool, sweet taste. it functions as a dusting agent with starch in chewing gum. it is used in sugarless candy, chewing gum, cereal, and pressed mints.
The Uses of Mannitol
Mannitol also known as wood mellow, is a kind of hexahydrin that can be used as a diuretic or sweetener.
Definition
A soluble hexahydric alcohol that occurs in many plants and fungi. It is used in medicines and as a sweetener (particularly in foods for diabetics). It is an isomer of sorbitol.
Definition
mannitol: A polyhydric alcohol,CH2OH(CHOH)4CH2OH, derived frommannose or fructose. It is the mainsoluble sugar in fungi and an importantcarbohydrate reserve in brownalgae. Mannitol is used as a sweetenerin certain foodstuffs and as a diureticto relieve fluid retention.
Production Methods
There are two main processes for industrial production of mannitol in the world. One is to take kelp as raw material. While producing alginate, the soaking solution of kelp after iodine extraction is obtained through multiple concentration, impurity removal, separation, evaporation concentration, cooling and crystallization; One is obtained from sucrose and glucose by hydrolysis, differential isomerization and enzyme isomerization, and then hydrogenation. China has used kelp to extract mannitol for decades. This process is simple and easy, but its development has been restricted for a long time due to the limitations of raw material resources, extraction yield, climatic conditions and energy consumption. The annual output of mannitol in China in the last century has never exceeded 8000 tons. The synthetic process in China began to be tested in the 1980s and came out in the 1990s. However, it has made great progress because it is not limited by raw materials and suitable for large-scale production.
Biotechnological Production
The by far largest quantity of mannitol is produced by chemical hydrogenation of
fructose which yields a mixture of mannitol and sorbitol. The mixture is subjected
to fractionated crystallization. As direct sorbitol production is less costly, the
processing costs have mostly to be borne by mannitol which makes it more
expensive than sorbitol. Production from seaweed seems to be of limited
importance.
Possibilities to produce mannitol by fermentation were studied using several
organisms. They mostly use fructose as an acceptor for hydrogen and glucose as a
source of carbon. In a fed-batch culture of C. magnoliae with 50 g/L of glucose as
the initial carbon source and increasing levels of fructose up to 300 g/L in 120 h, 248 g/L of mannitol were obtained from 300 g/L of fructose equivalent to a
conversion rate of 83 % and a productivity of 2.07 g/Lh.
High yields were obtained from Lactobacillus fermentum grown in a batch
reactor. The conversion rates increased from 25 to 35 C to 93.6 % with average
and high productivities of 7.6 and 16.0 g/Lh. A fast mannitol production of
104 g/L within 16 h was obtained from L. intermedius on molasses and fructose
syrups in a concentration of 150 g/L with a fructose-to-glucose rate of 4:1.
High productivity (26.2 g/Lh) and conversion rates (97 mol%) were obtained in a
high cell density membrane cell recycle bioreactor. Increase of the fructose concentration
above 100 g/L reduced the productivity. A fed-batch process with
L. intermedius yielded 176 g/L of mannitol from 184 g/L fructose and 94 g/L
glucose within 30 h. The productivity of 5.6 g/Lh could be increased to more than
40 g/Lh at the expense of reduced mannitol yield and increased residual substrate
concentrations.
As mannitol is more expensive than sorbitol, production by fermentation may
become an alternative to hydrogenation of fructose.
Pharmaceutical Applications
Mannitol is a good diuretic in medicine. It can reduce intracranial pressure, intraocular pressure, kidney medicine, dehydrating medicine, sugar substitute, excipient of tablets and diluent of solid and liquid. As a hypertonic antihypertensive drug, Injectio mannitou injection is commonly used in clinical rescue, especially in the rescue of brain diseases. It has the characteristics of fast antihypertensive and accurate curative effect required by drugs to reduce intracranial pressure. After mannitol enters the body, it can increase the plasma osmotic pressure, dehydrate the tissue, and reduce the intracranial pressure and intraocular pressure. After glomerular filtration, it is not easy to be reabsorbed by renal tubules, increase the urinary osmotic pressure, bring out a large amount of water and dehydrate. It is used for edema caused by craniocerebral trauma, brain tumor and brain tissue hypoxia, edema caused by large-area burn, ascites and glaucoma caused by renal failure. It can prevent and treat early acute renal insufficiency.
Mechanism of action
Today, mannitol is the most widely used osmotic diuretic. It raises osmotic pressure in renal tubules, thus reducing reabsorption of water in the nephrons. As a result, a large quantity of free water is released, sodium secretion increases, and as a rule, an insignificant amount of potassium is secreted. Mannitol is used as an adjuvant drug for preventing oliguria and anuriua.
Clinical Use
Mannitol is the agent most commonly used as an osmotic diuretic. Sorbitol also can be used for similar reasons.Mannitol is administered intravenously in solutions of 5 to 50% at a rate of administration that is adjusted to maintain the urinary output at 30 to 50 ml/hour. Mannitol is filtered at the glomerulus and is poorly reabsorbed by the kidney tubule. The osmotic effect of mannitol in the tubule inhibits the reabsorption of water, and the rate of urine flow can be maintained. It also is used to reduce intracranial pressure by reducing cerebral intravascular volume.
Side Effects
Large doses of mannitol used in treating cerebral edema can alter extracellular fluid volume, osmolality, and composition and can lead under some circumstances to acute renal insufficiency, cardiac decompensation, and other complications. The commonly recognized complications of osmotic agents used in patients with acute closed-angle glaucoma are mild headache, neck pain, nausea, and vomiting.
Safety Profile
A poison by intravenous route. Human systemic effects. When heated to decomposition it emits acrid smoke and irritating vapors.
Veterinary Drugs and Treatments
Mannitol is used to promote diuresis in acute oliguric renal failure, reduce intraocular and intracerebral pressures, enhance urinary excretion of some toxins, (e.g., aspirin, some barbiturates, bromides, ethylene glycol) and, in conjunction with other diuretics, to rapidly reduce edema or ascites when appropriate (see Contraindications- Precautions below). In humans, it is also used as an irrigating solution during transurethral prostatic resections.
Properties of Mannitol
| Melting point: | 167°C |
| Boiling point: | 494.9°C |
| Density | 1.596 |
| refractive index | 1.5970 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| pka | pKa 13.50(H2O,t =18)(Approximate) |
| form | Solid |
| color | White to off-white |
| Water Solubility | 177.3g/L(25 ºC) |
| Dielectric constant | 3.0(22℃) |
| InChI | InChI=1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4-,5-,6-/m1/s1 |
| CAS DataBase Reference | 87-78-5(CAS DataBase Reference) |
| EPA Substance Registry System | Mannitol (87-78-5) |
Safety information for Mannitol
Computed Descriptors for Mannitol
| InChIKey | FBPFZTCFMRRESA-KVTDHHQDSA-N |
| SMILES | OC[C@H]([C@H]([C@@H]([C@@H](CO)O)O)O)O |
Mannitol manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


![ALPHA-D-MAN-[1->2]-ALPHA-D-MAN-1->OME](https://img.chemicalbook.in/CAS/GIF/59571-75-4.gif)
![BETA-D-GLCNAC-[1->6]-ALPHA-D-MAN-1->OME](https://img.chemicalbook.in/CAS/GIF/80264-88-6.gif)
You may like
-
 87-78-5 Mannitol 98%View Details
87-78-5 Mannitol 98%View Details
87-78-5 -
 87-78-5 99%View Details
87-78-5 99%View Details
87-78-5 -
 Mannitol 87-78-5 99%View Details
Mannitol 87-78-5 99%View Details
87-78-5 -
 Mannitol CASView Details
Mannitol CASView Details -
 Mannitol Excipient GradeView Details
Mannitol Excipient GradeView Details
69-65-8 -
 Mannitol (69-65-8)View Details
Mannitol (69-65-8)View Details
69-65-8 -
 Mannitol Api PowderView Details
Mannitol Api PowderView Details
69-65-8 -
 ManinitolView Details
ManinitolView Details
87-78-5
