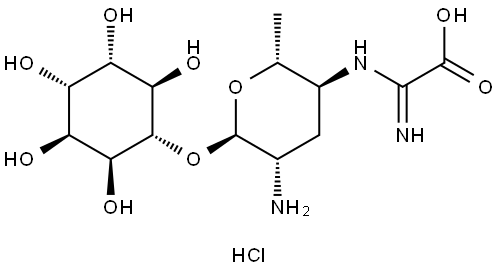
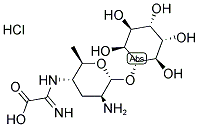
Kasugamycin hydrochloride
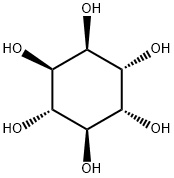
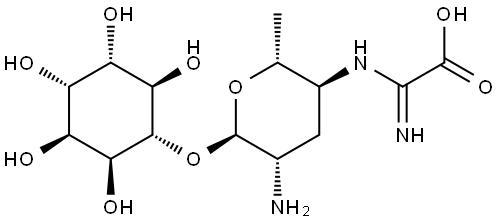
Synonym(s):3-O-[2-Amino-4-[(carboxyiminomethyl)amino]-2,3,4,6-tetradeoxy-D -arabino-hexopyranosyl]-D -chiro-inositol hydrochloride;3-O-[2-Amino-4-[(carboxyiminomethyl)amino]-2,3,4,6-tetradeoxy-D -arabino-hexopyranosyl]-D -chiro-inositol
- CAS NO.:19408-46-9
- Empirical Formula: C14H25N3O9.ClH
- Molecular Weight: 415.82
- MDL number: MFCD00058459
- EINECS: 637-144-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is Kasugamycin hydrochloride?
Description
Kasugamycin is an aminoglycosidic antibiotic isolated from S. kasugaensis. It blocks the initiation of translation, preventing initiation complex formation on 30S ribosomes in bacteria.
The Uses of Kasugamycin hydrochloride
Kasugamycin Hydrochloride is an aminoglycoside antibiotic that inhibits the initiation of translation. It is also a type of fungicide that controls leaf and neck blast disease caused by Pyricularia oryzae and increases rice yields.
The Uses of Kasugamycin hydrochloride
antifungal
The Uses of Kasugamycin hydrochloride
kasugamycin, derived from Streptomyces kasuguensis, is also used to control rice blast.It acts by inhibiting protein synthesis by interfering with the binding of aminoacyl-tRNA to the mRNA-30s ribosomal subunit complex (Yamaguchi, 1998).
What are the applications of Application
Kasugamycin hydrochloride is an antibiotic aminoglycoside that inhibits protein synthesis
in vitro
kasugamycin could inhibit protein synthesis by interacting with the 30s ribosomal subunit. the mechanism of inhibition of protein synthesis appeared to be different from that of other aminoglycosides, such as kanamycin, streptomycin, neomycin, gentamicin. the initiation complex formation on 30s ribosomes could be inhibited by kasugamycin but not by streptomycin, kanamycin or gentamicin, though the binding of fmet-trna to 70s ribosomes was inhibited by both streptomycin and by kasugamycin. [1].
in vivo
five groups, each consisting of five guinea pigs, were infected with shibaura strain; two groups were treated by injection with kasugamycin. all animals treated with 1 mcg/g were killed showing an average survival period of 7.4 days, which was close to the average survival period of 6.2 days in the control group; while all animals treated with 5 mcg/g survived. the other two groups were treated with kasugamycin from the icteric stage to the 10th day after infection. two animals of the 5 mcg/g group survived, showing no leptospira in the kidney; the other three died in an average of 7.7 days. three animals of the 10 mcg/g group survived; while the other two animals died in 6.5 days on an average . the total amounts of kasugamycin administered to the infected guinea pigs from the febrile stage were 10.8 mg (the 1 mcg/g group) and 54.0 mg (the 5 mcg/g group) [2].
Metabolic pathway
kasugamycin hydrochloride hydrate is a relatively stable antibiotic. There is limited published information on its metabolism and environmental fate but it is extensively degraded in plants and soil as indicated in Scheme 1.
Degradation
kasugamycin hydrochloride hydrate is very stable at room temperature. It is stable in weak acids but unstable in strong acids and under basic conditions. Its DT50 values at 50 °C are 47 and 14 days at pH 5 and 9, respectively (PM).
References
[1] okuyama, a. ,machiyama, n.,kinoshita, t., et al. inhibition by kasugamycin of initiation complex formation on 30s ribosomes. biochemical and biophysical research communications 43(1), 196-199 (1971).
[2] kitaoka m, mori m, arimitsu y. in vitro and in vivo effects of kasugamycin on leptospira icterohaemorrhagiae. jpn j med sci biol. 1975 oct-dec;28(5-6):285-90.
Properties of Kasugamycin hydrochloride
| Melting point: | 202-204 °C (decomp) |
| vapor pressure | <1.3x l0-8 Pa (25 °C) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | H2O: soluble |
| form | Solid |
| form | neat |
| Water Solubility | 125 g l-1 (25 °C) |
| pka | 3.23 (carboxyl), 7.73 and 11.0 (bases) |
| color | white to off-white |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 19408-46-9(CAS DataBase Reference) |
| EPA Substance Registry System | Kasugamycin monohydrochloride (19408-46-9) |
Safety information for Kasugamycin hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H320:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Kasugamycin hydrochloride
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Kasugamycin hydrochloride 90% (HPLC) CAS 19408-46-9View Details
Kasugamycin hydrochloride 90% (HPLC) CAS 19408-46-9View Details
19408-46-9 -
 Kasugamycin hydrochloride CAS 19408-46-9View Details
Kasugamycin hydrochloride CAS 19408-46-9View Details
19408-46-9 -
 Kasugamycin hydrochloride from Streptomyces kasugaensis CAS 19408-46-9View Details
Kasugamycin hydrochloride from Streptomyces kasugaensis CAS 19408-46-9View Details
19408-46-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1