Manganese nitrate
Synonym(s):Manganese dinitrate;Manganous nitrate
- CAS NO.:10377-66-9
- Empirical Formula: MnN2O6
- Molecular Weight: 178.95
- MDL number: MFCD00011112
- EINECS: 233-828-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
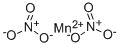
What is Manganese nitrate?
Description
Manganese nitrate is a colorless to pink crystalline solid. Molecular weight= 178.96. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 0, Reactivity 3 (Oxidizer). Highly soluble in water.
Chemical properties
Light red liquid
The Uses of Manganese nitrate
Ceramics, intermediates, catalyst, manganese dioxide.
The Uses of Manganese nitrate
Manganese(II) nitrate is used as a precursor to prepare the oxides of manganese. It is involved in the preparation of electronic components. Further, it is utilized for the phosphorous treatment of metal surface.
General Description
A white crystalline solid. Denser than water. May ignite on contact with organic matter. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Mixtures of metal/nonmetal nitrates with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures of nitrate with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. The anhydrous nitrate salt with urea explodes upon heating, [Chem Abs., 1975, 82, 48151].
Hazard
Fire and explosion risk in contact with organic materials.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
Mutation data reported. When heated to decomposition it emits toxic vapors of manganese.
Potential Exposure
Manganese nitrate is used as a color agent in porcelain and ceramic manufacture, as a catalyst, and in the production of manganese dioxide.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Medical observation is recommended for 2448 h after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a corticosteroid spray.
storage
Color Code—Yellow: Reactive Hazard (strong oxidizer); Store in a location separate from other materials, especially flammables and combustibles. Prior to working with this chemical you should be trained on its proper handling and storage. Manganese nitrate must be stored to avoid contact with organic materials since violent reactions occur. Store in tightly closed containers in a cool, well-ventilated area. Where possible, automatically transfer material from drums or other storage containers to process containers. See OSHA Standard 1910.104 and NFPA 43A Code for the Storage of Liquid and Solid Oxidizers for detailed handling and storage regulations.
Shipping
This compound requires a shipping label of “OXIDIZER.” It falls in Hazard Class 5.1 and Packing Group III.
Incompatibilities
A strong oxidizer. Violent reaction with reducing agents, organics, and combustible materials.
Properties of Manganese nitrate
| Melting point: | 37°C |
| Boiling point: | 100°C |
| Density | 1.536 g/mL at 25 °C |
| vapor pressure | 0Pa at 20℃ |
| RTECS | QU9780000 |
| solubility | very soluble in ethanol |
| form | Liquid |
| Specific Gravity | 1.5 |
| color | Pink |
| Water Solubility | soluble |
| Merck | 13,5756 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3 OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| CAS DataBase Reference | 10377-66-9(CAS DataBase Reference) |
| EPA Substance Registry System | Nitric acid, manganese(2+) salt (10377-66-9) |
Safety information for Manganese nitrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H314:Skin corrosion/irritation H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H373:Specific target organ toxicity, repeated exposure H402:Hazardous to the aquatic environment, acute hazard H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P220:Keep/Store away from clothing/…/combustible materials. P221:Take any precaution to avoid mixing with combustibles/… P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P311:Call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Manganese nitrate
| InChIKey | MIVBAHRSNUNMPP-UHFFFAOYSA-N |
Manganese nitrate manufacturer
Nithyasri Chemicals (APURVA CHEMICALS)
Monopoly Innovations Pvt. Ltd.
ARRAKIS INDUSTRIES LLP
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 10377-66-9 Manganese Nitrate 99%View Details
10377-66-9 Manganese Nitrate 99%View Details
10377-66-9 -
 10377-66-9 Manganese(II) nitrate 98%View Details
10377-66-9 Manganese(II) nitrate 98%View Details
10377-66-9 -
 Manganese(II) nitrate, 50% w/w aq. Soln CAS 10377-66-9View Details
Manganese(II) nitrate, 50% w/w aq. Soln CAS 10377-66-9View Details
10377-66-9 -
 Manganese(II) nitrate, 50% w/w aq. Soln CAS 10377-66-9View Details
Manganese(II) nitrate, 50% w/w aq. Soln CAS 10377-66-9View Details
10377-66-9 -
 Manganese nitrate 98.00% CAS 10377-66-9View Details
Manganese nitrate 98.00% CAS 10377-66-9View Details
10377-66-9 -
 MANGANESE (II) NITRATE SOLUTION (45-50%) 99%View Details
MANGANESE (II) NITRATE SOLUTION (45-50%) 99%View Details
10377-66-9 -
 Manganese nitrate, solution 45-50 wt.% CAS 10377-66-9View Details
Manganese nitrate, solution 45-50 wt.% CAS 10377-66-9View Details
10377-66-9 -
 MANGANESE NITRATE 45-50% SOLUTION IN DILUTE NITRIC ACID For Synthesis CAS 10377-66-9View Details
MANGANESE NITRATE 45-50% SOLUTION IN DILUTE NITRIC ACID For Synthesis CAS 10377-66-9View Details
10377-66-9