Maleic acid
Synonym(s):Butenedioic Acid;Toxilic acid;(2Z)-2-Butenedioic acid;(2Z)-But-2-enedioic acid;cis-Butenedioic acid
- CAS NO.:110-16-7
- Empirical Formula: C4H4O4
- Molecular Weight: 116.07
- MDL number: MFCD00063177
- EINECS: 203-742-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
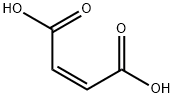
What is Maleic acid?
Description
Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCHCHCO2H. Maleic acid is the cis-isomer of butenedioic acid, where as fumaric acid is the trans-isomer. It is mainly used as a precursor to fumaric acid, and relative to its parent maleic anhydride, maleic acid has few applications.
Chemical properties
Maleic acid, also known as maleinic acid and toxilic acid, is a white crystalline (monoclinic) powder and possesses a faint acidulous odor and an astringent taste. It is soluble in water and alcohol. Maleic acid and fumaric acid are the simplest unsaturated carboxylic diacids. These acids experience two-step dissociation in aqueous solutions.They have the same structural formula but different spatial configurations. Fumaric acid is the trans and maleic acid the cis isomer. The physical properties of maleic acid and fumaric acid are very different. The cis isomer is less stable. Maleic acid is used in the preparation of fumaric acid by catalytic isomerization.
Physical properties
Maleic acid is a less stable molecule than fumaric acid. The difference in heat of combustion is 22.7 kJ·mol?1. The heat of combustion is -1355 kJ / mole. Maleic acid is more soluble in water than fumaric acid. The melting point of maleic acid (135 °C) is also much lower than that of fumaric acid (287 °C). Both properties of maleic acid can be explained on account of the intramolecular hydrogen bonding that takes place in maleic acid at the expense of intermolecular interactions, and that are not possible in fumaric acid for geometric reasons.
The Uses of Maleic acid
Maleic acid is used as a precursor to fumaric acid, dimethyl maleate and glyoxalic acid. It is an electrophile and acts as dienophine in Diels-Alder reactions. It reacts with drugs to form more stable addition salts like indacaterol maleate, carfenazine, chlorpheniramine, pyrilamine, methylergonovine and thiethylperazine. Its maleate ion is useful in biochemistry as an inhibitor of transaminase reactions.
Definition
ChEBI: Maleic acid is a butenedioic acid in which the double bond has cis- (Z)-configuration. It has a role as a plant metabolite, an algal metabolite and a mouse metabolite. It is a conjugate acid of a maleate(1-) and a maleate.
Production Methods
Maleic anhydride is the main source of maleic acid produced by hydration. Maleic anhydride is prepared commercially by the oxidation of benzene or by the reaction of butane with oxygen in the presence of a vanadium catalyst.
What are the applications of Application
Maleic acid is an industrial raw material for the production of glyoxylic acid by ozonolysis. It may be used to form acid addition salts with drugs to make them more stable, such as indacaterol maleate. Maleic acid is also used in manufacturing synthetic resins; in textile processing; in preserving oils and fats; to retard rancidity of fats and oils in 1:10,000 (these are said to keep 3 times longer than those without the acid); dyeing and finishing wool, cotton, and silk; preparing the maleate salts of antihistamines and similar drugs.
Reactions
Although not practised commercially, maleic acid can be converted into maleic anhydride by dehydration, to malic acid by hydration, and to succinic acid by hydrogenation (ethanol / palladium on carbon). It reacts with thionyl chloride or phosphorus pentachloride to give the maleic acid chloride (it is not possible to isolate the mono acid chloride). Maleic acid, being electrophilic, participates as a dienophile in many Diels - Alder reactions.
Synthesis Reference(s)
Journal of the American Chemical Society, 86, p. 4603, 1964 DOI: 10.1021/ja01075a017
The Journal of Organic Chemistry, 60, p. 6676, 1995 DOI: 10.1021/jo00126a013
Organic Syntheses, Coll. Vol. 2, p. 302, 1943
General Description
Maleic acid is a colorless crystalline solid having a faint odor. Maleic acid is combustible though Maleic acid may take some effort to ignite. Maleic acid is soluble in water. Maleic acid is used to make other chemicals and for dyeing and finishing naturally occurring fibers.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Maleic acid is a colorless to white crystalline solid. Moderately toxic. When heated to decomposition Maleic acid emits irritating fumes and acrid smoke [Lewis, 3rd ed., 1993, p. 790].
Hazard
Toxic by ingestion.
Health Hazard
Inhalation causes irritation of nose and throat. Contact with eyes or skin causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Irritating smoke containing maleic anhydride may form in fire.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Maleic acid is used in the pharmaceutical industry as a pH modifier and a buffering agent.It is also used to prevent rancidity of oils and fats; a ratio of 1 : 10 000 is usually sufficient to retard rancidity. Maleic acid is commonly used as a pharmaceutical intermediate to form the maleate salts of several categories of therapeutic agents, such as salts of antihistamines and other drug substances.
Safety Profile
Moderately toxic by ingestion and skin contact. Passes through intact skin. A skin and severe eye irritant and a corrosive. Believed to be more toxic than its isomer, fumeric acid. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Maleic acid is generally regarded as a nontoxic and nonirritant
material when used at low levels as an excipient. Maleic acid is used
in oral, topical, and parenteral pharmaceutical formulations in
addition to food products.
LD50 (mouse, oral): 2.40g/kg(7)
LD50 (rabbit, skin): 1.56g/kg
LD50 (rat, oral): 0.708g/kg
Potential Exposure
Maleic acid is used to make artificial resins, antihistamines, and to preserve (retard rancidity) of fats and oils
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24°48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Carcinogenicity
In chronic feeding studies, 12 Osborne–Mendel rats per group were fed 0.5, 1.0, or 1.5% maleic acid in their diets for 2 years. Concentrations of 1.0 and 1.5% maleic acid retarded the growth rate of rats, and all concentrations of maleic acid increased mortality rate; no tumorigenesis was reported. Toxicological differences from controls were not marked, and the pathology was nonspecific.
Storage
Maleic acid converts into the much higher-melting fumaric acid
(mp: 287°C) when heated to a temperature slightly above its melting
point.
Maleic acid is combustible when exposed to heat or flame. The
bulk material should be stored in airtight glass containers and
protected from light. It is recommended not to store it above 25°C.
Shipping
UN2215 Maleic acid, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Crystallise the acid from acetone/pet ether (b 60-80o) or hot water. Dry it at 100o. [Beilstein 2 H 748, 2 I 303, 2 II 641, 2 III 1911, 2 IV 2199.]
Incompatibilities
Dust may form explosive mixture with air, Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, amines, reducing agents; alkali metals
Incompatibilities
Maleic acid can react with oxidizing materials. Aqueous solutions are corrosive to carbon steels.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Liquid: incinerate after mixing with a flammable solvent. Use afterburner for complete combustion. Solid: dissolve in a flammable solvent or package in paper and burn. See above
Regulatory Status
Included in the FDA Inactive Ingredients Database (IM and IV injections; oral tablets and capsules; topical applications). Included in nonparenteral and parenteral medicines licensed in the UK.
Properties of Maleic acid
| Melting point: | 130-135 °C (lit.) |
| Boiling point: | 275°C |
| Density | 1.59 g/mL at 25 °C (lit.) |
| vapor pressure | 0.001Pa at 20℃ |
| refractive index | 1.5260 (estimate) |
| Flash point: | 127 °C |
| storage temp. | Store below +30°C. |
| solubility | 478.8g/l |
| form | Powder/Solid |
| pka | 1.83(at 25℃) |
| color | White |
| Specific Gravity | 1.59 |
| PH | 3.05(1 mM solution);2.21(10 mM solution);1.54(100 mM solution); |
| Water Solubility | 790 g/L (25 ºC) |
| Merck | 14,5703 |
| BRN | 605762 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, bases. |
| CAS DataBase Reference | 110-16-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Butenedioic acid (Z)-(110-16-7) |
| EPA Substance Registry System | Maleic acid (110-16-7) |
Safety information for Maleic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Maleic acid
| InChIKey | VZCYOOQTPOCHFL-OWOJBTEDSA-N |
Maleic acid manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Maleic Acid 99%View Details
Maleic Acid 99%View Details -
 110-16-7 98%View Details
110-16-7 98%View Details
110-16-7 -
 Maleic Acid CAS 110-16-7View Details
Maleic Acid CAS 110-16-7View Details
110-16-7 -
 Maleic Acid CASView Details
Maleic Acid CASView Details -
 Maleic Acid extrapure AR CAS 110-16-7View Details
Maleic Acid extrapure AR CAS 110-16-7View Details
110-16-7 -
 Maleic acid 95% CAS 110-16-7View Details
Maleic acid 95% CAS 110-16-7View Details
110-16-7 -
 Maleic Acid BP/USPView Details
Maleic Acid BP/USPView Details
110-16-7 -
 Maleic Acid BPView Details
Maleic Acid BPView Details
110-16-7
