Diethyl maleate
Synonym(s):Maleic acid diethyl ester
- CAS NO.:141-05-9
- Empirical Formula: C8H12O4
- Molecular Weight: 172.18
- MDL number: MFCD00009191
- EINECS: 205-451-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-04 13:31:24
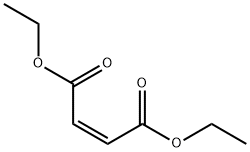
What is Diethyl maleate?
Chemical properties
Diethyl maleate is a colorless clear liquid between the temperatures of -10°C (pour point) and 225°C (boiling point). Insoluble in water, soluble in ethanol and ether. its odor type is fruity and its odor at 100% is described as "fruity bananacitrus". It is prohibited by lFRA and theEU because of sensitization and is notused anymore as fragrance.
Occurrence
Has apparently not been reported to occur in nature.
The Uses of Diethyl maleate
Diethyl maleate can be used for the preparation of organophosphorus insecticide malathion. It is unsaturated polyester resins that are used in applications, such as reinforced glass fiber for boats, piping, bathtubs, roof panels, etc., and as protective coatings, finishes, and lacquers.
The Uses of Diethyl maleate
Diethyl Maleate is used as a reagent in the synthesis of several organic compounds including that of polyaspartic acid ester based polyurea coatings. It was found to inhibit MCA and TPA transformed cell growth via modulation of glutathione, mitogen-activated protein kinase and cancer pathways.
What are the applications of Application
Diethylmaleate is a glutathione-depleting compound that inhibits NF kappa B
Preparation
Diethyl maleate is synthesized by the direct esterification of maleic anhydride and ethanol in the presence of sulfuric acid. Esterification is a commonly used reaction in organic synthesis.
Definition
ChEBI: Diethyl maleate is a maleate ester resulting from the formal condensation of both carboxy groups of maleic acid with ethanol. A colourless liquid at room temperature (m.p. -10℃) with boiling point 220℃ at 1 atm., it is commonly used as a dienophile for Diels-Alder-type cycloaddition reactions in organic synthesis. It has a role as a glutathione depleting agent. It is a maleate ester and an ethyl ester. It derives from an ethanol.
General Description
Diethyl maleate is reported to occur in the cabernet sauvignon wine.
Synthesis
5g maleic anhydride, 12g dehydrated alcohol, 0.3g acid zeolite, 15ml benzene are added to the reaction vessel, install water trap, reflux condensing tube, and thermometer on the reaction vessel, reflux water-dividing is carried out in heating, reaction reacts 30min again after occurring without the globule, stops heating, after normal temperature cooling 30min, release water layer, reaction solution washes with water, distill after drying, first steam front-end volatiles, the cut regathering 216 ~ 220 DEG C is product Diethyl maleate.
Metabolism
α/β-Unsaturated compounds, such as diethyl maleate, react enzymically with gluta thione. The reaction has been demonstrated in fractions of rat liver (Boyland & Chasseaud, 1967) and avian liver (Wit & Snel, 1968). The enzyme differs from other known S-alkyl, S-aryl and S- epoxide transferase enzymes responsible for glutathione-conjugate formation (Boyland & Chasseaud, 1967). Diethyl maleate, administered parenterally to rats, reduced the hepatic glutathione content (Boyland & Chasseaud, 1970; Varga, Fischer & Szily, 1974). The latter workers also showed that diethyl maleate pretreatment of rats inhibited the glutathione conjugation of subsequently-adminis tered bromsulphthalein.
Properties of Diethyl maleate
| Melting point: | -10 °C (lit.) |
| Boiling point: | 225 °C (lit.) |
| Density | 1.064 g/mL at 25 °C (lit.) |
| vapor density | 5.93 (vs air) |
| vapor pressure | 1 mm Hg ( 14 °C) |
| refractive index | n |
| Flash point: | 200 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 14mg/l |
| form | Liquid |
| color | Clear |
| Odor | at 100.00 %. fruity banana citrus |
| Water Solubility | insoluble |
| Merck | 14,3123 |
| BRN | 1100825 |
| Dielectric constant | 8.6(23℃) |
| Stability: | Stable. Combustible. Incompatible with oxidizing agents, bases, acids, reducing agents. |
| CAS DataBase Reference | 141-05-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Butenedioic acid (Z)-, diethyl ester(141-05-9) |
| EPA Substance Registry System | 2-Butenedioic acid (2Z)-, 1,4-diethyl ester (141-05-9) |
Safety information for Diethyl maleate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Diethyl maleate
| InChIKey | IEPRKVQEAMIZSS-AATRIKPKSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 141-05-9 Diethyl maleate 98%View Details
141-05-9 Diethyl maleate 98%View Details
141-05-9 -
 Diethyl Maleate CAS 141-05-9View Details
Diethyl Maleate CAS 141-05-9View Details
141-05-9 -
 Diethyl maleate CAS 141-05-9View Details
Diethyl maleate CAS 141-05-9View Details
141-05-9 -
 Diethyl maleate 95% CAS 141-05-9View Details
Diethyl maleate 95% CAS 141-05-9View Details
141-05-9 -
 Diethyl maleate CAS 141-05-9View Details
Diethyl maleate CAS 141-05-9View Details
141-05-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
