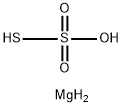
Magnesium thiosulfate hexahydrate
- CAS NO.:10124-53-5
- Empirical Formula: H4MgO3S2
- Molecular Weight: 140.45
- MDL number: MFCD00169871
- EINECS: 233-340-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
What is Magnesium thiosulfate hexahydrate?
Description
Magnesium thiosulfate has the molecular formula of
MgS2O3 and the molecular weight of 136.4352 g/mol.
It is also known as “hyposulfite”. It can be prepared
by the reaction of sodium thiosulfate upon the chloride
in water:
MgCl2+Na2S2O3? MgS2O3+2NaCl
Since magnesium thiosulfate is soluble in water
(50 g/100 ml at 20°C), the solution must be evaporated
at a low temperature to crystallize the product (salt-out
temperature ~15°F (-9.4°C). A hexahydrate, MgS2O3·
6H2O, is the result.
These are colorless crystals that lose water at 170°C to
form the trihydrate. Further heating to 420°C then
forms the anhydrate. Further heating to 600°C will
cause the production of magnesium sulfate, sulfur and
oxides of sulfur:
2MgS2O3+2O2+heat? 2MgSO4+S+SO2
Magnesium thiosulfate is considered to have a low
toxicity to humans. It has been used to remove ozone
from either a water stream, such as a drinking water
or wastewater stream being treated with ozone, or
scrubbing ozone from a gaseous stream by contacting
the gaseous stream with solid magnesium thiosulfate.
It has also been used to remove chlorine gas from a water
stream by contacting the stream with magnesium thiosulfate
solution. In yet another aspect, a method of
scrubbing chlorine from a gaseous stream comprises
contacting the gas stream with solid magnesium
thiosulfate.
Magnesium thiosulfate is not commonly referenced
in scientific literature. The few articles that prevail
discuss technically specific physical properties of the
end product itself. Synthetic methods are described in
some Japanese patents and there is mention of MgTS
in a short German description in Handbuch der Anorganischen
Chemie, vol. 11, 1984. Both refer to adding
sulfur to magnesium sulfite to produce magnesium
thiosulfate. Neither reference describes the pH nor
physical consistency of the “sulfite” intermediate,
although the German article does note the “color
change” observed as SO2 is purged into MgO: the slurry
becomes yellow.
Chemical properties
colorless or white crystal(s) [MER06]
Properties of Magnesium thiosulfate hexahydrate
| Melting point: | 1700°C |
| Density | 1.818 |
| Water Solubility | g/100g solution H2O: 30.69 (0°C), 34.51 (28°C); solid phase, MgS2O3 · 6H2O [KRU93]; insoluble alcohol [MER06] |
| Merck | 14,5694 |
| CAS DataBase Reference | 10124-53-5(CAS DataBase Reference) |
| EPA Substance Registry System | Thiosulfuric acid (H2S2O3), magnesium salt (1:1) (10124-53-5) |
Safety information for Magnesium thiosulfate hexahydrate
Computed Descriptors for Magnesium thiosulfate hexahydrate
Magnesium thiosulfate hexahydrate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 99% Magnesium Thiosulphate ArView Details
99% Magnesium Thiosulphate ArView Details
13446-30-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
