MAGNESIUM SULFIDE
- CAS NO.:12032-36-9
- Empirical Formula: MgS
- Molecular Weight: 56.37
- MDL number: MFCD00016207
- EINECS: 234-771-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
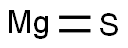
What is MAGNESIUM SULFIDE?
Chemical properties
Red-brown, crystalline solid.decomposes in water.
Chemical properties
Magnesium Sulfide is a red-brown, crystalline solid, decomposes above 2000 C, decomposes in water.
Physical properties
MgS is a white crystalline material but is often
encountered in an impure form that is a brown noncrystalline
powder.
The chemical properties of MgS resemble those of
related ionic sulfides such as those of Na, Ba, and Ca.
Magnesium Sulfide reacts with oxygen to form the corresponding sulfate,
magnesium sulfate. MgS reacts with water to give
hydrogen sulfide and magnesium hydroxide.
The Uses of MAGNESIUM SULFIDE
Magnesium Sulfide is the source of hydrogen sulfide, laboratory reagent.
Preparation
Magnesium Sulfide
can be prepared by the direct reaction of the elements,
calcined in an inert atmosphere, at a 1:1.1 molecular
ratio:
Mg+ S+ heat→MgS
MgS also forms by the reaction of hydrogen sulfide gas with soluble magnesium salts in solution.
Aside from being a component
of some slags, MgS is a rare nonterrestial mineral
“niningerite” detected in some meteorites.
Magnesium sulfide is available commercially from
a number of suppliers.
Properties of MAGNESIUM SULFIDE
| Melting point: | >2000°C (dec.) |
| Density | 2,84 g/cm3 |
| solubility | reacts with H2O |
| form | red-brown cubic crystals |
| color | red-brown cubic crystals, crystalline |
| Water Solubility | decomposes in H2O [HAW93] |
| EPA Substance Registry System | Magnesium sulfide (MgS) (12032-36-9) |
Safety information for MAGNESIUM SULFIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for MAGNESIUM SULFIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Magnesium sulphide CAS 12032-36-9View Details
Magnesium sulphide CAS 12032-36-9View Details
12032-36-9 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1