MAGNESIUM GLUCONATE, DIHYDRATE
- CAS NO.:59625-89-7
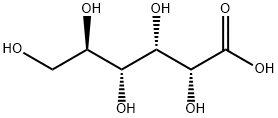
- Empirical Formula: C12H26MgO8
- Molecular Weight: 322.64
- MDL number: MFCD23379949
- EINECS: 222-848-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-21 22:41:43
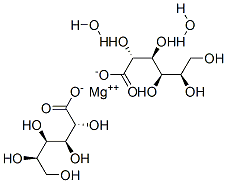
What is MAGNESIUM GLUCONATE, DIHYDRATE?
Absorption
A high-fat diet may decrease the amount of magnesium absorbed in the diet. Over-cooking food also may decrease the amount of magnesium absorbed from dietary sources .
About 1/3 of magnesium is absorbed from the small intestine. The fraction of magnesium absorbed is inversely proportional to amount ingested .
Oral absorption is estimated to be 15% to 30% .
Toxicity
Oral LD50 is 9100 mg/kg in the rat .
Excess magnesium from dietary sources does not pose a health risk in healthy individuals because the kidneys eliminate excess amounts of magnesium in the urine. On the other hand, high doses of magnesium from dietary supplements or medications often result in diarrhea that can be combined with nausea and abdominal cramping. Forms of magnesium most commonly reported to cause diarrhea include magnesium carbonate, chloride, gluconate, and oxide. Diarrheal and laxative effects of magnesium salts are due to the osmotic activity of unabsorbed salts in the intestine and colon and the stimulation of gastric motility .
Hypermagnesaemia after oral ingestion is uncommon except in patients with renal impairment. Signs and symptoms of hypermagnesemia may include respiratory depression, loss of deep tendon reflexes due to neuromuscular blockade, nausea, vomiting, flushing, hypotension, drowsiness, bradycardia and muscle weakness.
Very high doses of magnesium-containing laxatives and antacids (normally providing more than 5,000 mg/day magnesium) have been associated with the occurrence of magnesium toxicity, including fatal hypermagnesemia in a 28-month-old boy as well as an elderly man. Symptoms of magnesium toxicity, normally presenting at concentrations of 1.74–2.61 mmol/L, may include hypotension, nausea, vomiting, facial flushing, retention of urine, ileus, depression, and lethargy before progressing to muscle weakness, difficulty breathing, extreme hypotension, irregular heartbeat, and cardiac arrest. The risk of magnesium toxicity increases with compromised renal function or kidney failure because the ability to remove excess magnesium is reduced or lost .
Treatment: In patients with normal renal function, IV fluids or furosemide may be administered to promote the excretion of magnesium. In patients with symptomatic hypermagnesaemia, slow IV injection of calcium gluconate can be administered to antagonize the cardiac and neuromuscular effects of magnesium .
The Uses of MAGNESIUM GLUCONATE, DIHYDRATE
Replenisher (magnesium).
Background
Magnesium gluconate is a magnesium salt of gluconate. It demonstrates the highest oral bioavailability of magnesium salts and is used as a mineral supplement. Magnesium is ubiquitous in the human body, and is naturally present in many foods, added to other food products, available as a dietary supplement and used as an ingredient in some medicines (such as antacids and laxatives) .
Although magnesium is available in the form of sulphates, lactate, hydroxide, oxide and chloride, only magnesium gluconate is recommended for magnesium supplementation as it appears to be better absorbed and causes less diarrha .
This drug has been studied in the prevention of pregnancy-induced hypertension, and has displayed promising results . In addition, it has been studied for its effects on premature uterine contractions .
Indications
Magnesium gluconate is a mineral supplement which is used to prevent and treat low levels of magnesium. Magnesium is very important for the normal physiologic functioning of cells, nerves, muscles, bones, and the heart. Generally, a well-balanced diet provides the necessary amounts of magnesium for homeostasis. However, certain conditions causing chronic magnesium deficiency may decrease levels of magnesium. These conditions include treatment with diuretics, a poor diet, alcoholism, or other medical conditions (e.g., severe diarrhea/vomiting, stomach/intestinal absorption problems, poorly controlled diabetes) .
brand name
Almora(Forest).
Pharmacokinetics
Magnesium is a cofactor in over 300 enzyme systems that regulate a variety of biochemical reactions in the body, including protein synthesis, muscle and nerve function, blood glucose control, and blood pressure regulation. Magnesium is necessary for energy production, oxidative phosphorylation, and glycolysis .
Metabolism
Not Available
Properties of MAGNESIUM GLUCONATE, DIHYDRATE
| Melting point: | 200 °C (dec.)(lit.) |
Safety information for MAGNESIUM GLUCONATE, DIHYDRATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for MAGNESIUM GLUCONATE, DIHYDRATE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran

You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5