magnesium aluminosilicate
- CAS NO.:71205-22-6
- Empirical Formula: AlH11MgO5Si
- Molecular Weight: 170.45
- MDL number: MFCD00864493
- EINECS: 000-000-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-03 16:17:04
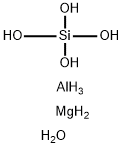
What is magnesium aluminosilicate?
Absorption
In regards to aluminum-containing antacids like the aluminum-magnesium complex almasilate, it is reported that the bioavailability of ingested aluminum from such antacids is only between 0.01-1% . Additionally, while the optimal absorption of magnesium appears to be in the small intestine and particularly in the ileum and jejunum, with some absorption in the colon, it is believed that the transfer of magnesium from blood to extravascular space is quick and efficient .
Toxicity
Readily accessible data regarding the toxicity of almasilate is not available.
Originator
Megalac Almasilat ,Krewel Meuselbach
Indications
Almasilate is indicated for use as an antacid for the neutralization of excess stomach acid . It is subsequently also used for the symptomatic treatment of diseases where it is necessary to neutralize acid in the stomach, for example, for treating stomach and duodenal ulcers or heartburn and stomach conditions caused by excess stomach acid .
Background
Almasilate is a buffering antacid that has been used in peptic ulcers and dyspepsia. It is a crystalline polyhydrate of aluminium/magnesium silicate and mediates its buffering activity by binding hydrogen ions within the polymer. However its therapeutic efficacy is not comparable to other approved antacids, as it is no more effective in neutralizing acid and binding bile salts than other conventional antacids .
Given that there are generally more widely available conventional antacids that are just as - if not more - effective than almasilate, almasilate products are only available in certain parts of Europe and/or Asia.
Manufacturing Process
203 g of crystalline magnesium chloride such as is used as a food additive,
containing 46% MgCl2, is dissolved in 600 ml of water, to which 96 g of
caustic soda dissolved in 250 ml of water is added with stirring and a solution
comprising 50 ml of water with 207 g of sodium silicate (Na2O, 9%, SiO2,
29%) is further added and is then vigorously stirred to produce basic sodium
magnesium silicate (which is designated as Slurry A).
Secondly 593 g of aluminum sulfate containing 17.2% Al2O3, dissolved in
1,700 ml of water is gently added with stirring to 216 g of caustic soda
dissolved in 600 ml of water, to which 50 ml of water added to 207 g of
sodium silicate (Na2O 9%, SiO2, 29%) is slowly added and is then vigorously
stirred to produce tetrabasic dialuminum silicate (which is designated as
Slurry B). Slurry A and Slurry B are mixed up vigorously with stirring for 3
hours at room temperature. The thus obtained white gel-like precipitate is
washed by decantation to remove free alkali, sodium sulfate, sodium chloride,
etc. produced as reaction by-products. The residue is filtered and dried at
105°-110°C and 340 g of white powder of fine particle size is obtained as the
final product. The molar ratio of MgO to Al2O3, to SiO2, in this product is
approximately 1: 1: 2.
203 g of crystalline magnesium chloride such as is used as a food additive,
containing 46% MgCl2, and 593 g of aluminum sulfate containing 17.2%
Al2O3, are dissolved in 2,300 ml of water, to which a solution comprising 272
g of caustic soda with 800 ml of water, is slowly added with stirring.
Thereafter, a solution comprising 414 g of sodium silicate (Na2O, 9%, SiO2,
29%) with 100 ml of water is added, the mixture is heated and is stirred for
five hours, while keeping the temperature at 60°C. When the reaction mixture
becomes neutral it is allowed to cool and to stand, the supernatant fluid is
withdrawn and the white gel-like precipitate is washed by decantation to
remove the impurities and dried at 105°-110°C and 350 g of white powder of
fine particle size is obtained as the final product (almasilate).
Therapeutic Function
Antacid
Pharmacokinetics
Various non-prescription antacid products are available to the general public to purchase and use as a treatment for relieving the occasional, limited, and non-severe sensation of heartburn (or acid indigestion) that may frequently be associated with stomach indigestion (or dyspepsia) or gastroesophageal reflux disease (when stomach contents may rise back up into the esophagus and cause a taste of acid in the back of the mouth, among other symptoms) .
Subsequently, when excessive amounts of acids are produced in the stomach (either owing to indigestion or acid reflux, for example), the natural mucous barrier protecting the lining of the stomach can be damaged . Antacids like almasilate consequently contain alkaline ions that chemically neutralize such stomach gastric acids, providing relief to heartburn, relieving pain, and reducing damage in the area .
Metabolism
Once aluminum has entered the body, the mechanism by which it is metabolized is still not fully known . Conversely, magnesium balance in the body is predominantly managed via a balance between intestinal absorption, exchange with bone, and renal excretion .
Properties of magnesium aluminosilicate
| CAS DataBase Reference | 71205-22-6 |
Safety information for magnesium aluminosilicate
Computed Descriptors for magnesium aluminosilicate
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1