m-Anisidine
Synonym(s):3-Aminoanisole;3-Methoxyaniline
- CAS NO.:536-90-3
- Empirical Formula: C7H9NO
- Molecular Weight: 123.15
- MDL number: MFCD00007783
- EINECS: 208-651-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
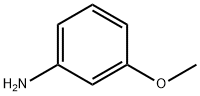
What is m-Anisidine?
Description
Anisidine exists as ortho-, meta-, and paraisomers. They have characteristic amine (fishy) odors.o-isomer: A colorless to pink liquid. Solid below 5℃.Molecular weight= 123.17; Boiling point= 224℃;Melting/Freezing point= 5℃; Vapor pressure=,0.1 mmHg at 20℃; Flash point= 118℃ (oc);Autoignition temperature= 415℃. Hazard Identification(based on NFPA-704 M Rating System): Health 2,Flammability 1, Reactivity 0. Practically insoluble in water;solubility=1.5%.m-isomer: Pale yellow liquid. Molecular weight= 123.2;Boiling point= 251℃; Freezing/Melting point= 57.2℃;Vapor pressure= 0.006 mmHg at 20℃.p-isomer: Reddish-brown solid. Molecular weight= 123.2;Boiling point= 243℃; Freezing/Melting point= 57.2℃;Vapor pressure: 0.4 mmHg at 20℃; Flash point= 122℃;Autoignition temperature= 515℃. Hazard Identification(based on NFPA-704 M Rating System): Health 2,Flammability 0, Reactivity 0. Insoluble in water.
Chemical properties
Light yellow oily liquid with have characteristic amine (fishy) odors. soluble in alcohol, ether, benzene and dilute acid, slightly soluble in water. Anisidine exists as ortho-, meta-, and paraisomers.
The Uses of m-Anisidine
m-Anisidine is a highly poisonous monosubstituted aniline used as corrosion inhibitorsfor aluminum, copper and other metals in acidic solutions.
The Uses of m-Anisidine
The unusually large amount of dibromo product produced upon bromination of m-anisidine may be attributed to the two doubly activeated positions. The best enantioselectivity of 97 % ee was observed for the reaction of m-anisidine in organocatalytic asymmetric three-component cyclization of cinnamaldehydes and primary amines with 1, 3-Dicarbonyl Compounds. Evidence for the control of 2nd-harmonic generation activities from the x-ray crystal-structures of the complexes of l-tartaric acid with m-anisidine and p-toluidine was determined.
What are the applications of Application
m-Anisidine is used in:
The synthesis of N-substituted-3-chloro-2-azetidinones, which are potential anthelmintic agents.
Rhodium-catalyzed synthesis of indoles and copper-catalyzed synthesis of benzimidazoles.
In the preparation of azocalix[4]arene dyes.
Preparation
m-Aminoanisole is synthesized by reduction of m-nitrophenol after methylation on the hydroxyl group.
A mixture of 35 g. (0.23 mole) of m-nitroanisole (p. 213), 110 ml. of methanol, and 7.5 ml. of concentrated hydrochloric acid is stirred and heated to boiling. Forty-two grams (0.75 gram atom) of iron filings is added in small portions over a 1-hour period, and refluxing and stirring are continued for 5 additional hours. The mixture is made strongly alkaline with sodium hydroxide and steam-distilled, the methanol which first distils over being collected separately. The remainder of the distillate is extracted with ether; the ethereal solu-tion is dried over anhydrous sodium sulfate and distilled. m-Anisidine (23.2 g. or 80%) is collected at 125°/13 mm.
Reference: J. Chem. Soc, 1934, 1420; J. Chem. Soc, 127, 494 (1925).
Definition
ChEBI: 3-Methoxyaniline is a substituted aniline and an aromatic ether.
Synthesis Reference(s)
The Journal of Organic Chemistry, 22, p. 333, 1957 DOI: 10.1021/jo01354a610
Tetrahedron Letters, 24, p. 4121, 1983 DOI: 10.1016/S0040-4039(00)88277-5
General Description
M-anisidine appears as pale yellow oily liquid or dark red liquid. (NTP, 1992)
Air & Water Reactions
m-Anisidine may be sensitive to prolonged exposure to air and light. Insoluble in water.
Reactivity Profile
m-Anisidine is incompatible with strong oxidizers. m-Anisidine is also incompatible with acids, acid chlorides, acid anhydrides and chloroformates.
Fire Hazard
m-Anisidine is probably combustible.
Safety Profile
Moderately toxic by ingestion.Mutation data reported. When heated to decomposition itemits toxic vapors of NOx.
Potential Exposure
Anisidines are used in the manufacture of azo dyes; pharmaceuticals; textile-processing chemicals Incompatibilities: Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine,bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks some coatings and some forms of plastic and rubber.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immedi ately with soap and water. Seek medical attentionAnisidines 231immediately. If this chemical has been inhaled, removefrom exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine.
Storage
Color Code—Green: General storage. Prior toworking with this chemical you should be trained on itsproper handling and storage. Store in tightly closed containers in a cool, dark, well-ventilated area. Protect againstsunlight and strong oxidizers. Metal containers involvingthe transfer of this chemical should be grounded andbonded. Where possible, automatically pump liquid fromdrums or other storage containers to process containers.Drums must be equipped with self-closing valves, pressurevacuum bungs, and flame arresters. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of this chemical. Sources of ignition, such assmoking and open flames, are prohibited where this chemical is used, handled, or stored in a manner that could create a potential fire or explosion hazard. A regulated,marked area should be established where this chemical ishandled, used, or stored in compliance with OSHAStandard 1910.1045.
Shipping
UN2431 Anisidines, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
o-Isomer impurity can be removed by steam distillation. Another possible impurity is the precursor 3-nitroanisole which can be removed as for the preceding o-isomer and fractionating using an efficient column. It is a yellow liquid. [Gilman & Kyle J Am Chem Soc 74 3027 1952, Bryson J Am Chem Soc 82 4858 1960, Kadaba & Massie J Org Chem 22 333 1957, Beilstein 13 IV 953.]
Incompatibilities
Incompatible with strong oxidizers, withrisk of fire or explosions. Attacks some coatings and someforms of plastic and rubber.
Waste Disposal
Dissolve in combustible solvent (alcohols, benzene, etc.) and spray solution into furnace equipped with afterburner and scrubber, or burn spill residue on sand and soda ash absorbent in a furnace.
Properties of m-Anisidine
| Melting point: | −1-1 °C(lit.) |
| Boiling point: | 251 °C(lit.) |
| Density | 1.096 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | 18g/l |
| form | Liquid |
| pka | 4.23(at 25℃) |
| color | Clear yellow to brown |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| Sensitive | Light Sensitive |
| Merck | 14,667 |
| BRN | 386119 |
| CAS DataBase Reference | 536-90-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzenamine, 3-methoxy-(536-90-3) |
| EPA Substance Registry System | m-Anisidine (536-90-3) |
Safety information for m-Anisidine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for m-Anisidine
| InChIKey | NCBZRJODKRCREW-UHFFFAOYSA-N |
m-Anisidine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 m-Anisidine, 98% 99%View Details
m-Anisidine, 98% 99%View Details
536-90-3 -
 m-Anisidine CAS 536-90-3View Details
m-Anisidine CAS 536-90-3View Details
536-90-3 -
 m-Anisidine 97% CAS 536-90-3View Details
m-Anisidine 97% CAS 536-90-3View Details
536-90-3 -
 m-Anisidine CAS 536-90-3View Details
m-Anisidine CAS 536-90-3View Details
536-90-3 -
 m-ANISIDINE For Synthesis CAS 536-90-3View Details
m-ANISIDINE For Synthesis CAS 536-90-3View Details
536-90-3 -
 m-Anisidine CAS 536-90-3View Details
m-Anisidine CAS 536-90-3View Details
536-90-3 -
 3-Aminoanisole CAS Number 536-90-3View Details
3-Aminoanisole CAS Number 536-90-3View Details
536-90-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
