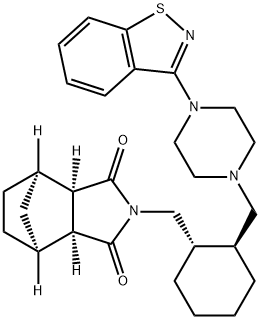
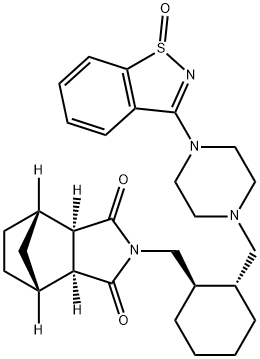
lurasidone
- CAS NO.:367514-87-2
- Empirical Formula: C28H36N4O2S
- Molecular Weight: 492.68
- MDL number: MFCD14635357
- EINECS: 696-042-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
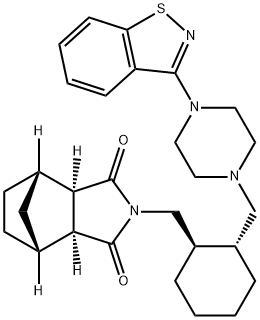
What is lurasidone?
Absorption
Lurasidone is readily absorbed and quickly reaches maximal concentrations (Cmax) within 1-4 hours. When taken with food, there is a two-fold increase in exposure and time to maximal concentration is increased by 0.5-1.5 hours. This occurs regardless of fat or caloric content. Bioavailability = 9-19%.
Description
The atypical antipsychotic lurasidone (also known as SM-13496) was approved in the United States in 2010 as an oral agent for the treatment of patients with schizophrenia. Lurasidone has potent affinity for D2 (Ki= 1.7 nM) and 5-HT2A (Ki= 2.0 nM) receptors and acts as an antagonist at both receptors. It is also a partial agonist at the 5-HT1A receptor and, unlike other atypical agents, is a potent antagonist at the 5-HT7 receptor; both of these activities are thought to confer beneficial cognitive properties. Lurasidone is further differentiated by its lack of affinity for muscarinic and histamine H1 receptors and its weak affinity for the 5-HT2C receptor. Antagonism at H1 and 5-HT2C receptors has been implicated in weight gain associated with atypical agents, while muscarinic receptor antagonism is associated with cognitive deficits.
Originator
Dainippon Sumitomo Pharma (Japan)
Background
Lurasidone is an atypical antipsychotic developed by Dainippon Sumitomo Pharma. It was approved by the U.S. Food and Drug Administration (FDA) for treatment of schizophrenia on October 29, 2010 and is currently pending approval for the treatment of bipolar disorder in the United States.
Indications
Lurasidone is indicated for the treatment of schizophrenia in patients ≥13 years old. It is also indicated as a monotherapy for the treatment of bipolar depression in patients ≥10 years old, or in combination with lithium or valproate for the treatment of bipolar depression in adults.
Definition
ChEBI: An N-arylpiperazine that is (3aR,4S,7R,7aS)-2-{[(1R,2R)-2-(piperazin-1-ylmethyl)cyclohexyl]methyl}hexahydro-1H-4,7-m thanoisoindole-1,3(2H)-dione in which position N4 of the piperazine ring is substituted by a 1,2-benzothiazol-3-yl group. Lurasidone is used (generally as the hydrochloride salt) as an atypical antipsychotic for the treatment of schizoph enia.
brand name
Latuda
Pharmacokinetics
Lurasidone is a benzothiazol derivative that is an antagonist and binds with high affinity to Dopamine-2 (D2) (Ki = 0.994 nM), 5-HT2A (Ki = 0.47 nM) receptors, and 5-HT7 receptors (Ki = 0.495 nM). It also binds with moderate affinity to alpha-2C adrenergic receptors (Ki = 10.8 nM) and is a partial agonist at 5-HT1A receptors (Ki = 6.38 nM). Its actions on histaminergic and muscarinic receptors are negligible.
Metabolism
Lurasidone is metabolized by CYP3A4 in which its major active metabolite is referred to as ID-14283 (25% of parent exposure). Its two minor metabolites are referred to as ID14326 and ID11614 which make up 3% and 1% of parent exposure respectively. Its two non-active metabolites are referred to as ID-20219 and ID-20220.
Properties of lurasidone
| Melting point: | 146-149°C |
| Boiling point: | 623.4±55.0 °C(Predicted) |
| Density | 1.273 |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 8.41±0.50(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 367514-87-2 |
Safety information for lurasidone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for lurasidone
| InChIKey | PQXKDMSYBGKCJA-CVTJIBDQSA-N |
| SMILES | C1(=O)[C@]2([H])[C@@]([H])([C@]3([H])C[C@@]2([H])CC3)C(=O)N1C[C@@H]1CCCC[C@H]1CN1CCN(C2C3=C(SN=2)C=CC=C3)CC1 |
lurasidone manufacturer
Megafine Pharma
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
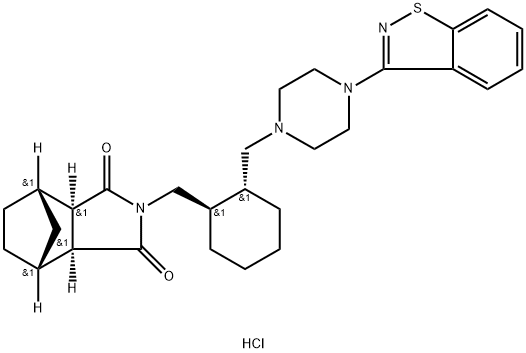
You may like
-
 367514-87-2 Lurasidone 98%View Details
367514-87-2 Lurasidone 98%View Details
367514-87-2 -
 367514-87-2 96%View Details
367514-87-2 96%View Details
367514-87-2 -
 Lurasidone 367514-87-2 98%View Details
Lurasidone 367514-87-2 98%View Details
367514-87-2 -
 Lurasidone 98%View Details
Lurasidone 98%View Details
367514-87-2 -
 367514-87-2 98%View Details
367514-87-2 98%View Details
367514-87-2 -
 367514-87-2 Lurasidone 99%View Details
367514-87-2 Lurasidone 99%View Details
367514-87-2 -
 Lurasidone 99% (HPLC) CAS 367514-87-2View Details
Lurasidone 99% (HPLC) CAS 367514-87-2View Details
367514-87-2 -
 Lurasidone 99%View Details
Lurasidone 99%View Details
367514-87-2