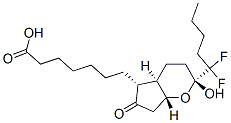
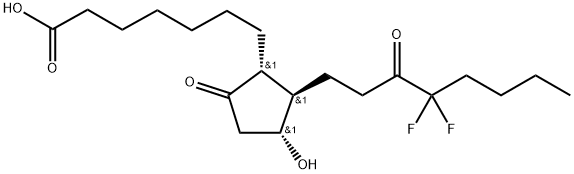
Lubiprostone
Synonym(s):(11a,15R)-11,15-Epoxy-16,16-difluoro-15-hydroxy-9-oxo-prostan-1-oic acid;Ru 0211;Spi 0211
- CAS NO.:136790-76-6
- Empirical Formula: C20H32F2O5
- Molecular Weight: 390.47
- MDL number: MFCD20268389
- EINECS: 634-172-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Lubiprostone?
Absorption
Lubiprostone has low systemic availability following oral administration and concentrations of lubiprostone in plasma are below the level of quantitation (10 pg/mL).
Toxicity
In a definitive Phase 1 cardiac repolarization study, 51 patients were administered a single oral dose of 144 mcg of lubiprostone, which is 6 times the normal single administration dose. Thirty-nine (39) of the 51 patients experienced an adverse event. The adverse events reported in >1% of this group included the following: nausea (45.1%), vomiting (27.5%), diarrhea (25.5%), dizziness (17.6%), loose or watery stools (13.7%), headache (11.8%), retching (7.8%), abdominal pain (5.9%), flushing or hot flush (5.9%), dyspnea (3.9%), pallor (3.9%), stomach discomfort (3.9%), syncope (3.9%), upper abdominal pain (2.0%), anorexia (2.0%), asthenia (2.0%), chest discomfort (2.0%), dry mouth (2.0%), hyperhidrosis (2.0%), skin irritation (2.0%) and vasovagal episode (2.0%).
Chemical properties
Lubiprostone is a white, odorless crystals or crystalline powder and is very soluble in ether and ethanol, and practically insoluble in hexane and water. It is an activator of ClC-2 chloride channels, used in the management of idiopathic chronic constipation.
The Uses of Lubiprostone
Lubiprostone is a bicyclic fatty acid metabolite analog of Prostaglandin E1. It activates specific chloride channels in the gastrointestinal tract to stimulate intestinal fluid secretion, increase gastrointestinal transit, and improve symptoms of constipation.
Background
Lubiprostone is a medication used in the management of idiopathic chronic constipation. A prostaglandin E1 derivative, lubiprostone is a bicyclic fatty acid that activates ClC-2 chloride channels located on the apical side of the gastrointestinal epithelial cells. Activation of these channels promotes the secretion of a chloride-rich fluid that soften the stool, increase gastrointestinal motility, and induce spontaneous bowel movements (SBM).
Indications
Lubiprostone is indicated for the treatment of adult patients with chronic idiopathic constipation, or opioid-induced constipation in patients with chronic non-cancer pain. It is also indicated for the treatment of irritable bowel syndrome with constipation (IBS-C) in female patients ≥18 years old.
What are the applications of Application
Lubiprostone is a prostaglandin E1 metabolite
What are the applications of Application
Lubiprostone is a locally acting chloride channel activator for the treatment of gastrointestinal disorders.
Biochem/physiol Actions
Lubiprostone is a bicyclic fatty acid that activates ClC-2 and CFTR chloride channels. Some reports suggest that lubiprostone opens CFTR or both CFTR and ClC-2 through interactions with the prostaglandin receptor EP4. Lubiprostone is used clinically to treat idiopathic chronic constipation and irritible bowel syndrome.
Pharmacokinetics
Chronic idiopathic constipation is generally defined by infrequent or difficult passage of stool. The signs and symptoms associated with chronic idiopathic constipation (i.e., abdominal pain or discomfort, bloating, straining, and hard or lumpy stools) may be the result of abnormal colonic motility that can delay the transit of intestinal contents and impede the evacuation of rectal contents. One approach to the treatment of chronic idiopathic constipation is the secretion of fluid into the abdominal lumen through the activation of chloride channels in the apical membrane of the gastrointestinal epithelium. Lubiprostone is a locally acting chloride channel activator that increases intestinal chloride and fluid secretion without altering sodium and potassium concentrations in the serum.
Metabolism
The results of both human and animal studies indicate that lubiprostone is rapidly and extensively metabolized by 15-position reduction, α-chain β-oxidation, and ω-chain ω-oxidation. These biotransformations are not mediated by the hepatic cytochrome P450 system but rather appear to be mediated by the ubiquitously expressed carbonyl reductase. M3, a metabolite of lubiprostone in both humans and animals is formed by the reduction of the carbonyl group at the 15-hydroxy moiety that consists of both α-hydroxy and β-hydroxy epimers. M3 makes up less than 10% of the dose of radiolabeled lubiprostone.
Properties of Lubiprostone
| Melting point: | 56-59°C |
| Boiling point: | 539.1±50.0 °C(Predicted) |
| Density | 1.143±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 4.77±0.10(Predicted) |
| form | powder |
| color | white to beige |
Safety information for Lubiprostone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lubiprostone
Lubiprostone manufacturer
Honour Lab Limited
BDR Pharmaceuticals International Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 136790-76-6 Lubiprostone 98%View Details
136790-76-6 Lubiprostone 98%View Details
136790-76-6 -
 Lubiprostone 98%View Details
Lubiprostone 98%View Details
136790-76-6 -
 136790-76-6 Lubiprostone 98%View Details
136790-76-6 Lubiprostone 98%View Details
136790-76-6 -
 136790-76-6 98%View Details
136790-76-6 98%View Details
136790-76-6 -
 Lubiprostone 98%View Details
Lubiprostone 98%View Details
136790-76-6 -
 Lubiprostone 136790-76-6 98%View Details
Lubiprostone 136790-76-6 98%View Details
136790-76-6 -
 Lubiprostone 98.00% CAS 136790-76-6View Details
Lubiprostone 98.00% CAS 136790-76-6View Details
136790-76-6 -
 Lubiprostone CAS 136790-76-6View Details
Lubiprostone CAS 136790-76-6View Details
136790-76-6