LOXO-292
- CAS NO.:2152628-33-4
- Empirical Formula: C29H31N7O3
- Molecular Weight: 525.6
- MDL number: MFCD31814089
- EINECS: 207-854-9
- Update Date: 2024-11-19 23:02:33
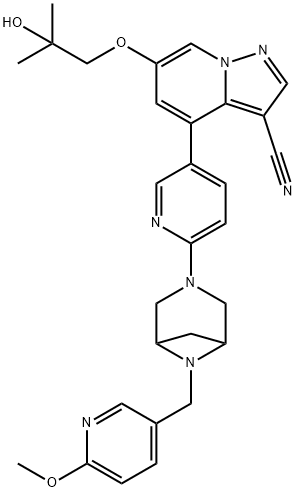
What is LOXO-292?
Absorption
In patients with locally advanced or metastatic solid tumours receiving 160 mg of selpercatinib twice daily, steady-state was achieved after approximately 7 days, with a Cmax of 2,980 (CV 53%) and AUC0-24h of 51,600 (CV 58%). The absolute bioavailability is between 60 and 82% (mean 73%), and the median tmax is two hours. Food has no apparent effect on the AUC or Cmax of selpercatinib. Patients with hepatic impairment display a concomitant increase in AUC0-INF for mild (7%), moderate (32%), and severe (77%) impairment.
Toxicity
Toxicity information regarding selpercatinib is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as hepatotoxicity, hypertension, prolonged QT interval, and hemorrhaging. Symptomatic and supportive measures are recommended.
The Uses of LOXO-292
Selpercatinib is a small molecule protein kinase inhibitor which may be used in the treatment of cancer.
Indications
Selpercatinib is approved to treat:
Selpercatinib is currently approved for these indications under an accelerated approval scheme and continued approval may be contingent on future confirmatory trials.
Background
Selpercatinib is a kinase inhibitor with enhanced specificity for RET tyrosine kinase receptors (RTKs) over other RTK classes. Enhanced RET (Rearranged during transfection) oncogene expression is a hallmark of many cancers. Although multikinase inhibitors, including cabozantinib, ponatinib, sorafenib, sunitinib, and vandetanib, have shown efficacy in RET-driven cancers, their lack of specificity is generally associated with substantial toxicity. Selpercatinib (LOXO-292) and pralsetinib (BLU-667) represent the first generation of specific RET RTK inhibitors for the treatment of RET-driven cancers.
Although selpercatinib is currently still under investigation in clinical trial NCT04211337, it was granted accelerated FDA approval on May 8, 2020, for specific RET-driven cancer indications. It is currently marketed under the brand name RETEVMO? by Loxo Oncology Inc. Selpercatinib is also approved by the European Commission.
brand name
Retevmo
General Description
Class: receptor tyrosine kinase; Treatment: RET-altered lung/thyroid cancers; Other name: ARRY-192, LOXO-292; Oral bioavailability = 73%; Elimination half-life = 32 h; Protein binding = 97%
Pharmacokinetics
Selpercatinib exerts anti-tumour activity in specific cancers through inhibition of mutated forms of RET tyrosine kinases. Due to its increased specificity for RET over other tyrosine kinases, selpercatinib is thought to have an improved safety profile compared to other multi-kinase inhibitors. Despite this, selpercatinib treatment is associated with hepatotoxicity, hypertension, QT interval prolongation, hemorrhagic events, risk of impaired wound healing, and embryo-fetal toxicity; some patients may also exhibit hypersensitivity to selpercatinib.
Side Effects
Selpercatinib can cause serious side effects including liver toxicity, high blood pressure, heart rhythm changes due to prolongation of heart electrical activity (QT prolongation), bleeding, allergic reactions, impaired wound healing and harm to an unborn baby.
Selpercatinib may cause harm to a developing fetus or a newborn baby.
Metabolism
Selpercatinib is predominantly metabolized in the liver by CYP3A4.
storage
Store at -20°C
Dosage
The recommended selpercatinib dose based on body weight is:
(1) Less than 50 kg: 120 mg orally twice daily
(2) 50 kg or greater: 160 mg orally twice daily
Properties of LOXO-292
| Density | 1.36±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:20.0(Max Conc. mg/mL);38.05(Max Conc. mM) |
| form | A crystalline solid |
| pka | 14.00±0.29(Predicted) |
| color | White to off-white |
Safety information for LOXO-292
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for LOXO-292
| InChIKey | XIIOFHFUYBLOLW-UHFFFAOYSA-N |
| SMILES | C12=C(C#N)C=NN1C=C(OCC(O)(C)C)C=C2C1=CC=C(N2CC3CC(N3CC3=CC=C(OC)N=C3)C2)N=C1 |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4