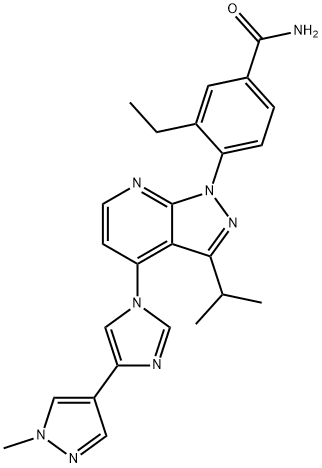
Futibatinib
- CAS NO.:1448169-71-8
- Empirical Formula: C22H22N6O3
- Molecular Weight: 418.45
- MDL number: MFCD29037352
- Update Date: 2024-07-02 08:54:57
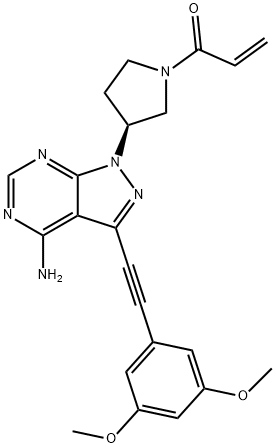
What is Futibatinib?
Absorption
Tmax ranges from 1.2 to 22.8 hours, with a median value of two hours. In healthy subjects, a high-fat and high-calorie meal (900 to 1000 calories with approximately 50% of total caloric content from fat) decreased futibatinib AUC by 11% and Cmax by 42%.
Toxicity
There is no information available regarding acute toxicity and overdose of futibatinib.
The Uses of Futibatinib
Futibatinib is an orally bioavailable inhibitor of the fibroblast growth factor receptor (FGFR) with potential antineoplastic activity. Futibatinib selectively and irreversibly binds to and inhibits FGFR, which may result in the inhibition of both the FGFR-mediated signal transduction pathway and tumor cell proliferation, and increased cell death in FGFR-overexpressing tumor cells. FGFR is a receptor tyrosine kinase essential to tumor cell proliferation, differentiation and survival and its expression is upregulated in many tumor cell types.
Background
Futibatinib is an inhibitor of Fibroblast Growth Factor receptor (FGFR), which comprises a group of receptor tyrosine kinases that play a key role in cell proliferation, differentiation, migration, and survival. FGFR was investigated in oncology as a therapeutic target, as FGFR genomic aberrations and dysregulated FGFR signalling pathways are observed in some cancers such as cholangiocarcinoma and urothelial malignancies.
As a novel inhibitor of FGFR, futibatinib was first approved by the FDA in September 2022 to treat different types of intrahepatic cholangiocarcinoma. On July 4, 2023, the European Commission granted Conditional Marketing Authorization for futibatinib for the treatment of cholangiocarcinoma.
Indications
Futibatinib is indicated to treat adults with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma harbouring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements. In Europe, it is indicated in patients whose disease has progressed after at least one prior line of systemic therapy.
Futibatinib is approved in the US under accelerated approval and in Europe under conditional marketing authorization. This currently approved indication is subject to change, as it may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
brand name
Lytgobi
General Description
Class: receptor tyrosine kinase; Treatment: Cholangiocarcinoma; Other name: TAS 120; Elimination half-life = 2.9 h; Protein binding = 95%
Pharmacokinetics
Futibatinib is an anticancer agent with demonstrated anti-tumour activity in mouse and rat xenograft models of human tumours with activating FGFR genetic alterations. Futibatinib is not expected to affect cell lines with no FGFR genomic aberrations. It suppresses the growth of tumours in a dose-dependent manner.
Synthesis
The synthesis of Futibatinib is as follows:
2 methanesulfonate (25.0 g), water (69 mL), and acetonitrile (158 mL) were placed in a reaction vessel, and a 5N aqueous sodium hydroxide solution (35 mL) was added thereto. A solution prepared by diluting 3-chloropropionyl chloride (6.27 g) with acetonitrile (50 mL) was added over a period of 10 minutes. After completion of the dropwise addition, the mixture was stirred at 30° C. for 30 minutes. The reaction solution was partially taken out and measured by HPLC (condition 3). The peak area of compound B was confirmed to be less than 0.1% of the total peak area. At this stage, the diamide compound and the 3CP diamide compound were not detected in HPLC. Thereafter, a 5N aqueous sodium hydroxide solution (25 mL) was further added, and the mixture was stirred at 30° C. for 4 hours. The reaction solution was partially taken out and measured by HPLC (condition 3). The peak area of the A-1-3CP compound was confirmed to be less than 0.1% of the total peak area. After completion of the reaction, water (550 mL) was added over a period of 2 hours. After completion of the dropwise addition, the internal temperature was adjusted to 25° C., and the mixture was stirred for 1.5 hours. The insoluble matter was collected by filtration and washed with water (125 mL), followed by drying the washed matter at 60° C. under reduced pressure, thereby obtaining the Futibatinib (16.02 g, yield 85.3%). 
in vitro
Futibatinib (TAS-120) covalently binds to a highly conserved P-loop cysteine residue in the ATP pocket of FGFR.
in vivo
Futibatinib (TAS-120) (3, 30, 100 mg/kg/day, p.o.) exerts an anti-tumor effect in mice. Futibatinib (TAS-120) shows anti-tumor effect by administering at moderate intervals, such as intermittent administration of every other day dosing and 2 times/week, and reducing the sustained elevation and weight suppression blood phosphorus level, and take a antitumor effective as daily administration.
Metabolism
In vitro, futibatinib is primarily metabolized by CYP3A and to a lesser extent by CYP2C9 and CYP2D6. Unchanged futibatinib is the major drug-related moiety in plasma (accounting for 59% of radioactivity) in healthy subjects.
Properties of Futibatinib
| Boiling point: | 733.8±60.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:38.0(Max Conc. mg/mL);90.81(Max Conc. mM) DMF:30.0(Max Conc. mg/mL);71.69(Max Conc. mM) DMF:PBS (pH 7.2) (1:5):0.16(Max Conc. mg/mL);0.38(Max Conc. mM) Ethanol:0.5(Max Conc. mg/mL);1.19(Max Conc. mM) |
| form | A crystalline solid |
| pka | 3.60±0.30(Predicted) |
| color | White to yellow |
Safety information for Futibatinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Futibatinib
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4