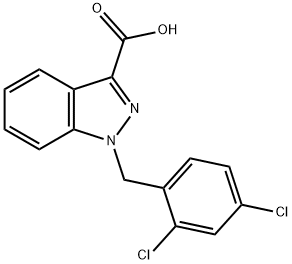
Lonidamine
Synonym(s):1-(2,4-Dichlorobenzyl)-1H-indazole-3-carboxylic acid;Diclondazolic acid
- CAS NO.:50264-69-2
- Empirical Formula: C15H10Cl2N2O2
- Molecular Weight: 321.16
- MDL number: MFCD00866285
- EINECS: 256-510-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Lonidamine?
Description
Lonidamine is an antineoplastic agent reportedly effective for the treatment of various cancers, including lung, breast, prostate and brain tumors. Its clinical effect appears to be associated with changes in cellular energy metabolism.
Originator
Angelini (Italy)
The Uses of Lonidamine
contraceptive, spermicidal
What are the applications of Application
Lonidamine is it elicits cell apoptosis and blocks CFTR Cl- channel
Definition
ChEBI: A member of the class of indazoles that is 1H-indazole that is substituted at positions 1 and 3 by 2,4-dichlorobenzyl and carboxy groups, respectively.
brand name
Doridamina
Biological Activity
Anticancer and antispermatogenic agent in vitro and in vivo . Inhibits cellular energy metabolism in some cells via inhibition of mitochondrial hexokinase. Also blocks CFTR Cl - channels in vitro .
Biochem/physiol Actions
Inhibits the energy metabolism of neoplastic cells by interfering with hexokinase and disrupting uncoupler-stimulated mitochondrial electron transport; damages cell and mitochondrial membranes.
storage
Room temperature
References
1) Gatto?et al. (2002),?Recent studies on lonidamine, the lead compound of the antispermatogenic indazol-carboxylic acids; Contraception,?65?277 2) Floridi?et al., (1981),?Effect of Lonidamine on the Energy Metabolism of Ehrlich Ascites Tumor Cells; Cancer Res.,?41?4661 3) Ravagnan?et al. (1999),?Lonidamine triggers apoptosis via a direct, Bcl-2-inhibited effect on the mitochondrial permeability transition pore; Oncogene,?18?2537 4) Ben-Horin?et al. (1995),?Mechanism of Action of the Antineoplastic Drug Lonidamine: 31P and 13C Nuclear Magnetic Resonance Studies; Cancer Res.?55?2814 5) Nath?et al. (2016),?Mechanism of antineoplastic activity of lonidamine; Biochim.Biophys.Acta Reviews on Cancer?1866?151
Properties of Lonidamine
| Melting point: | 207-209°C |
| Boiling point: | 537.9±45.0 °C(Predicted) |
| Density | 1.4835 (rough estimate) |
| refractive index | 1.6070 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Soluble in DMSO (up to 25 mg/ml). |
| form | solid |
| pka | 3.00±0.10(Predicted) |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| CAS DataBase Reference | 50264-69-2(CAS DataBase Reference) |
Safety information for Lonidamine
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H351:Carcinogenicity H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Lonidamine
| InChIKey | WDRYRZXSPDWGEB-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Lonidamine CAS 50264-69-2View Details
Lonidamine CAS 50264-69-2View Details
50264-69-2 -
 Lonidamine 97.00% CAS 50264-69-2View Details
Lonidamine 97.00% CAS 50264-69-2View Details
50264-69-2 -
 Lonidamine CAS 50264-69-2View Details
Lonidamine CAS 50264-69-2View Details
50264-69-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8