Lofexidine
- CAS NO.:31036-80-3
- Empirical Formula: C11H12Cl2N2O
- Molecular Weight: 259.13
- MDL number: MFCD00865915
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
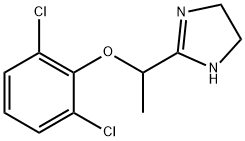
What is Lofexidine?
Absorption
Lofexidine has a good oral bioavailability and the peak plasma concentration occurs after 2-5 hours of oral administration. The bioavailability is registered to be even higher than 72%. About 30% of the administered dose of lofexidine is lost during first-pass metabolism. The absorption is registered to be very rapidly recirculated in the gut. After oral administration of 0.8 mg of lofexidine, a maximal dose of 1.26 ng/ml is achieved after 3 hours.
Toxicity
Lofexidine did not exhibit genotoxic, mutagenic nor mutagenic potential. Administration at gestational period showed a reduction in the neonatal weight, survival, and increased abortion.
The Uses of Lofexidine
An tihypertensive.
Background
Lofexidine is a non-opioid centrally acting alpha2-adrenergic receptor agonist that was first approved for the treatment of opioid withdrawal in the United Kingdom in 1992. It was first studied for use as an antihypertensive in 1980, but its researched was stopped as it was found less effective for the treatment of hypertension than clonidine. Lofexidine was then repurposed for the treatment of opioid withdrawal, as it was seen to be more economical and have fewer side effects than clonidine. Lofexidine was developed by US Woldmeds LLC and it was approved by the FDA on May 16, 2018.
Indications
Lofexidine is indicated for mitigation of symptoms associated with acute withdrawal from opioids and for facilitation of the completion of opioid discontinuation treatment. It is the first non-opioid medication for the symptomatic management of opioid discontinuation.
Opioid withdrawal syndrome is a debilitating manifestation of opioid dependence. This condition is extremely unpleasant lasting several days with some of the main features being abdominal pain, nausea, diarrhea, mydriasis, lacrimation, and piloerection. These symptoms are often observed after abrupt reductions in the opioid dose and can be resolved by re-administration of the opioid.
Definition
ChEBI: Lofexidine is a member of imidazoles, a dichlorobenzene, an aromatic ether and a carboxamidine. It has a role as an alpha-adrenergic agonist and an antihypertensive agent.
brand name
Lofexidine (Rhone-Poulenc Rorer).
Pharmacokinetics
In clinical trials, lofexidine presented more severe opioid withdrawal effects than observed with methadone. On the other hand, in clinical trials of methadone withdrawal, lofexidine effectively reduced withdrawal symptoms, especially hypotension. The clinical reports have also indicated that lofexidine presents a better outcome when used briefly. In phase 3 clinical trials, lofexidine was shown to generate a significantly higher completion rate of opioid discontinuation. Some pharmacological studies were performed and there were no off-target effects reported.
Metabolism
Lofexidine metabolic ratio is highly variable among people. It is metabolized mainly by the activity of CYP2D6 and in a minor degree by CYP1A2 and CYP2C19. These enzymes catalyze the hydroxylation of lofexidine and the opening of imidazoline ring to form N-(2-aminoethyl)-2-(2,6-dichlorophenoxy)propanamide. This metabolite is deamidated and forms 2-(2,6-dichlorophenoxy) propionic acid and 2,6-dichlorophenol. These three main metabolites are inactive.
Properties of Lofexidine
| Melting point: | 126-128° |
| Boiling point: | 421.5±35.0 °C(Predicted) |
| Density | 1.39±0.1 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | DMSO: 62.5 mg/mL (241.19 mM) |
| pka | 9.80±0.40(Predicted) |
| form | Solid |
| color | White to off-white |
| CAS DataBase Reference | 31036-80-3(CAS DataBase Reference) |
Safety information for Lofexidine
Computed Descriptors for Lofexidine
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
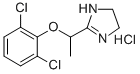

You may like
-
 Lofexidine 95% CAS 31036-80-3View Details
Lofexidine 95% CAS 31036-80-3View Details
31036-80-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8