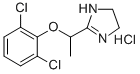
Lofexidine hydrochloride
Synonym(s):2-[1-(2,6-Dichlorophenoxy)ethyl]-4,5-dihydro-1H-imidazole hydrochloride;Britlofex;Lofetensin;Loxacor;
- CAS NO.:21498-08-8
- Empirical Formula: C11H13Cl3N2O
- Molecular Weight: 295.59
- MDL number: MFCD00917022
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-01-02 15:08:17
What is Lofexidine hydrochloride?
Chemical properties
Crystalline Solid
Originator
Lofetensin,Nattermann,W. Germany,1981
The Uses of Lofexidine hydrochloride
Lofexidine hydrochloride is an α2-adrenergic receptor agonist (Kd = 7.6 nM for rat cerebral cortex membranes) that has transient antihypertensive effects. It is used in managing opioid withdrawal symptoms during detoxification from heroin and methadone.
The Uses of Lofexidine hydrochloride
a2-Adrenoceptor agonist related structurally to Clonidine. Used in treatment of opioid withdrawal symptoms; antihypertensive.
The Uses of Lofexidine hydrochloride
α2-Adrenoceptor agonist related structurally to Clonidine. Used in treatment of opioid withdrawal symptoms; antihypertensive.
Manufacturing Process
10.4 ml of absolute ethanol are added to 57.5g of α-2,6-
dichlorophenoxypropionitrile, followed by the introduction of 100 ml of
chloroform dried over phosphorus pentoxide; 10.4 g of carefully dried
hydrogen chloride being slowly introduced with stirring and cooling with
ice/common salt. Most of the chloroform and excess hydrogen chloride is then
removed by filtration in vacuo at room temperature, and dry ether added to
the residue until the imido acid ester hydrochloride is quantitatively
precipitated. The α-dichlorophenoxypropionimido acid ethyl ester
hydrochloride can be obtained analytically pure in the form of white, strongly
hygroscopic crystals by repeated dissolution in a little absolute ethanol in the
absence of heat, and precipitation with ether.
The crude α-(2,6-dichlorophenoxy)propionamido acid ethyl ester hydrochloride
is added in portions to a stirred, ice-cooled solution of 29.5 g of anhydrous
ethylenediamine in 200 ml of absolute ethanol in such a way that the
temperature does not exceed 0°C to 5°C. The cooling bath is then removed
and the reaction mixture heated for 1 hour on a water bath to approximately
70°C.
After cooling, unreacted ethylenediamine is neutralized in a cooling mixture
with the absolute ethanolic hydrochloric acid, filtered off from any components
that are insoluble in ethanol and approximately two-thirds of the solvent
filtered off under suction in a water jet pump vacuum. Residual quantities ofethylenediamine dihydrochloride are precipitated in fractions by the careful
addition of ethyl methyl ketone, after which the imidazoline hydrochloride is
separated off by the addition of dry ether. Following repeated recrystallization
from ethanol ether, 2-[α-(2,6-dichlorophenoxy)ethyl]-δ2-imidazoline
hydrochloride is obtained in the form of small white crystals melting at 221°C
to 223°C.
brand name
Lofexidine (Rhone-Poulenc Rorer).
Therapeutic Function
Antihypertensive
Biological Activity
lofexidine is a α2-receptor agonist for opioid detoxification. lofexidine shows a strong affinity for the α2a-receptor subtype [1].the α2 adrenergic receptor is a g protein-coupled receptor (gpcr) consisting three highly homologous subtypes, α2a-, α2b-, and α2c-adrenergic. the α-receptors in brain are important presynaptic modulators of central noradrenergic function (autoreceptors) and postsynaptic mediators of many effects of catecholamines and related drugs. the α2-adrenergic agonists can be used as antihypertensives and preanesthetic agents [2].lofexidine is extensively absorbed, reaching peak concentrations at approximately 3 hours after oral administration. the mean maximum serum concentrations following a single 1.2 or 2.0 mg dose in healthy male adults were 1755 ± 306 and 2795 ± 593 ng/ml, respectively [1].
References
[1] gish e c, miller j l, honey b l, et al. lofexidine, an α2-receptor agonist for opioid detoxification[j]. annals of pharmacotherapy, 2010, 44(2): 343-351.
[2] scheinin m, lomasney j w, hayden-hixson d m, et al. distribution of α2-adrenergic receptor subtype gene expression in rat brain[j]. molecular brain research, 1994, 21(1): 133-149.
Properties of Lofexidine hydrochloride
| Melting point: | 230-232°C |
| storage temp. | -20°C Freezer |
| solubility | H2O: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| InChI | InChI=1S/C11H12Cl2N2O.ClH/c1-7(11-14-5-6-15-11)16-10-8(12)3-2-4-9(10)13;/h2-4,7H,5-6H2,1H3,(H,14,15);1H |
| CAS DataBase Reference | 21498-08-8(CAS DataBase Reference) |
Safety information for Lofexidine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Lofexidine hydrochloride
| InChIKey | DWWHMKBNNNZGHF-UHFFFAOYSA-N |
| SMILES | C1(=C(Cl)C=CC=C1Cl)OC(C)C1=NCCN1.Cl |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Lofexidine hydrochloride 98.00% CAS 21498-08-8View Details
Lofexidine hydrochloride 98.00% CAS 21498-08-8View Details
21498-08-8 -
 Lofexidine hydrochloride 95% CAS 21498-08-8View Details
Lofexidine hydrochloride 95% CAS 21498-08-8View Details
21498-08-8 -
 Lofexidine hydrochloride CAS 21498-08-8View Details
Lofexidine hydrochloride CAS 21498-08-8View Details
21498-08-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1