Lithium Triethylborohydride
Synonym(s):Lithium triethylborohydride solution
- CAS NO.:22560-16-3
- Empirical Formula: C6H13BLi
- Molecular Weight: 102.92
- MDL number: MFCD00011703
- EINECS: 245-076-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
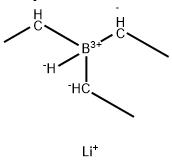
What is Lithium Triethylborohydride?
Description
Lithium triethylborohydride is the organoboron compound with the formula LiEt3BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid but is typically marketed and used as a THF solution. The related reducing agent sodium triethylborohydride is commercially available as toluene solutions.
LiBHEt3 is a stronger reducing agent than lithium borohydride and lithium aluminium hydride.
Chemical properties
colorless to light grey cloudy solution
The Uses of Lithium Triethylborohydride
Powerful and selective reducing agent.
As a reducing agentLithium triethylborohydride is used as a powerful reducing agent in organic synthesis for the conversion of carbonyl compounds to alcohols. It is also used in the preparation of alkynyl alcohols from the cleavage of cyclic keto-vinyl triflates. It is also used in the reduction of esters and lactones to alcohol and diol respectively. Further, it is used to prepare 1-methylcyclohexanol and 1,4-butanediol from 1,2-epoxybutane and gamma-butyrolactone respectively. It is also utilized for reductive cleavage of mesylates and tosylates in synthetic organic chemistry.
The Uses of Lithium Triethylborohydride
LiTEBH can be used as a reagent:
- To reduce alkyl halides to alkanes via dehydrogenation reactions.
- For the selective reduction of epoxides to Markovnikov alcohols.
- To reduce tosylates or mesylates primary alcohols to hydrocarbons.
- For reductive cyclization reactions for the preparation of useful intermediates.
- In the synthesis of hepta(manno-3-deoxy-6-O-t-butyldimethylsilyl)-β-cyclodextrin by reduction of hepta(manno-2,3-anhydro-6-O-t-butyldimethylsilyl)-β-cyclodextrin.
- For hydrodefluorination of C-F bonds using Ni catalyst.
- To prepare alkynyl alcohols from cleavage of cyclic keto-vinyl triflates.
- In the stereoselective reduction of bicyclic imides, isoquinolines, and pyridines.
- To prepare tungsten and molybdenum hydride complexes.
What are the applications of Application
Lithium Triethylborohydride (LiTEBH) is widely used as a powerful and selective reducing agent that shows super hydride activity in organic synthesis.
LiTEBH can be used as a reagent:
To reduce alkyl halides to alkanes via dehydrogenation reactions.
For the selective reduction of epoxides to Markovnikov alcohols.
To reduce tosylates or mesylates primary alcohols to hydrocarbons.
For reductive cyclization reactions for the preparation of useful intermediates.
In the synthesis of hepta(manno-3-deoxy-6-O-t-butyldimethylsilyl)-β-cyclodextrin by reduction of hepta(manno-2,3-anhydro-6-O-t-butyldimethylsilyl)-β-cyclodextrin.
For hydrodefluorination of C-F bonds using Ni catalyst.
To prepare alkynyl alcohols from cleavage of cyclic keto-vinyl triflates.
In the stereoselective reduction of bicyclic imides, isoquinolines, and pyridines.
To prepare tungsten and molybdenum hydride complexes.
Preparation
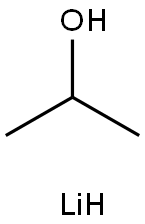
LiBHEt3 is prepared by the reaction of lithium hydride (LiH) and triethylborane (Et3B) in tetrahydrofuran (THF):
LiH + Et3B → LiEt3BH
Its THF solutions are stable indefinitely in the absence of moisture and air.
General Description
Super-Hydride? solution (Lithium triethylborohydride or LiTEBH) is widely used as a powerful and selective reducing agent that shows super hydride activity in organic synthesis.
Precautions
Moisture sensitive. Air sensitive. Incompatible with water and strong oxidizing agents.
References
Tamang, S.; Kim, K.; Choi, H.; Kim, Y.; Jeong, S. Synthesis of colloidal InSb nanocrystals via in situ activation of InCl3. Dalton Trans. 2015, 44 (38), 16923-16928.
Kandapallil, B.; Colborn, R. E.; Bonitatibus, P. J.; Johnson, F. Synthesis of high magnetization Fe and FeCo nanoparticles by high temperature chemical reduction. J. Magn. Magn. Mater. 2015, 378, 535-538.
Properties of Lithium Triethylborohydride
| Melting point: | 66-67° (Binger); mp 78-83° (dec) (Brown, 1977) |
| Density | 0.892 g/mL at 25 °C |
| Flash point: | 1 °F |
| storage temp. | Flammables + water-Freezer (-20°C)e area |
| solubility | Miscible with tetrahydrofuran and benzene. |
| form | Liquid |
| color | Colorless to light gray |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,5544 |
| BRN | 4151240 |
| Exposure limits | ACGIH: TWA 50 ppm; STEL 100 ppm (Skin) OSHA: TWA 200 ppm(590 mg/m3) NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) |
| CAS DataBase Reference | 22560-16-3(CAS DataBase Reference) |
Safety information for Lithium Triethylborohydride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H302:Acute toxicity,oral H314:Skin corrosion/irritation H318:Serious eye damage/eye irritation H333:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P405:Store locked up. P402+P404:Store in a dry place. Store in a closed container. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Lithium Triethylborohydride
Lithium Triethylborohydride manufacturer
JSK Chemicals
HYCHEM LABORATORIES
New Products
Tetrabutylammonium iodide (3,3-DIFLUOROCYCLOBUTYL)METHANOL 4,4-DIFLUOROCYCLOHEXANAMINE Cyclobutylamine (S)-3-Fluoro-pyrrolidine hydrochloride 3-Oxocyclobutanecarboxylic acid N-Hydroxy-2-methylpropanimidamide L-tert-Leucine,97% 2-Bromophenylacetonitrile, 97% Aluminum oxide, basic Calcium hydroxide, 95% Diallylamine, 99% 2-Iodobenzoic Acid 3-Methoxybenzonitrile Pentachlorobenzonitrile Chloral Dibenzoyl Peroxide Titanium Dioxide O-Benzylhydroxylamine Hydrochloride 2-Nitrobenzaldehyde 2-Picolylamine (2-Aminomethylpyridine) 2-Venylpyridine Ethyl-2-Chloroacetoacetate 4-Dimethylamine PyridineRelated products of tetrahydrofuran

![LITHIUM TRIETHYLBOROHYDRIDE, [3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB8412362.gif)
You may like
-
 Lithium triethylborohydride 98%View Details
Lithium triethylborohydride 98%View Details
22560-16-3 -
 LITHIUM TRIETHYLBOROHYDRIDE (1.0 M in THF) 22560-16-3View Details
LITHIUM TRIETHYLBOROHYDRIDE (1.0 M in THF) 22560-16-3View Details
22560-16-3 -
 Lithium triethylborohydride, 1M Solution in THF CAS 22560-16-3View Details
Lithium triethylborohydride, 1M Solution in THF CAS 22560-16-3View Details
22560-16-3 -
 Lithium triethylhydridoborate CAS 22560-16-3View Details
Lithium triethylhydridoborate CAS 22560-16-3View Details
22560-16-3 -
 Lithium triethylborohydride, 1M in THF solution CAS 22560-16-3View Details
Lithium triethylborohydride, 1M in THF solution CAS 22560-16-3View Details
22560-16-3 -
 Lithium triethylhydridoborate, 1M in TH 99%View Details
Lithium triethylhydridoborate, 1M in TH 99%View Details
22560-16-3 -
 Lithium tri-ethyl borohydride 20% in THF CAS 22560-16-3View Details
Lithium tri-ethyl borohydride 20% in THF CAS 22560-16-3View Details
22560-16-3 -
 Super-Hydride® solution CAS 22560-16-3View Details
Super-Hydride® solution CAS 22560-16-3View Details
22560-16-3
