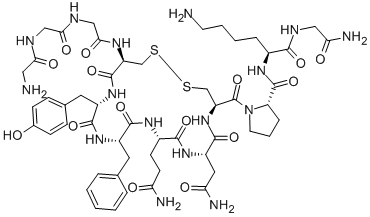
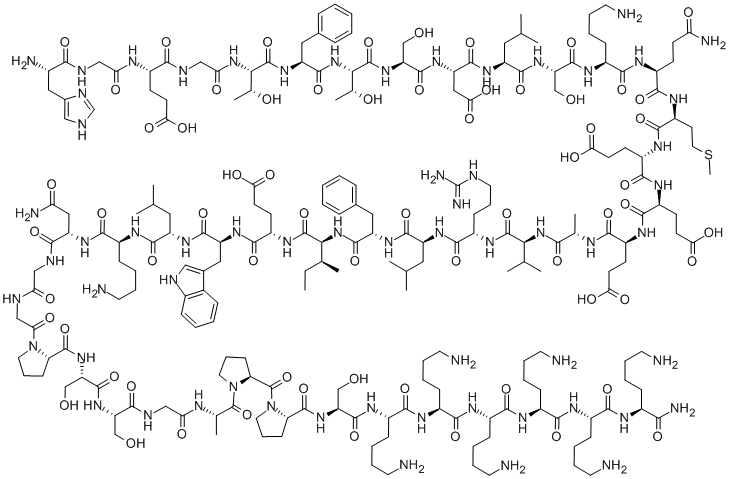
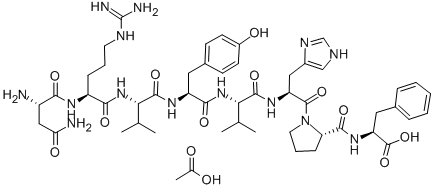
Teriparatide acetate
Synonym(s):Parathormone (1-34);Parathyroid Hormone 1-34, Human - CAS 52232-67-4 - Calbiochem;PTH 1-34;Teriparatide
- CAS NO.:52232-67-4
- Empirical Formula: C172H278N52O47S2
- Molecular Weight: 3890.49792
- MDL number: MFCD00149013
- EINECS: 640-978-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-31 13:32:20
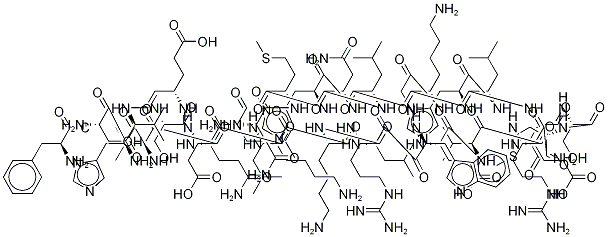
What is Teriparatide acetate?
The Uses of Teriparatide acetate
A fragment of human parathyroid hormone (hPTH) peptide sequence containing the 34 N-terminal residues of hPTH. This fragment was also found to be an agonist at PTH1 and PTH2 receptors.
General Description
Teriparatide is a recombinantform of parathyroid hormone, which is used for the treatmentof osteoporosis in men and postmenopausal women.The N-terminal region possesses 34 amino acids, which areidentical to the biologically active region of the 84-aminoacid sequence of human parathyroid hormone. It has beenshown to act on osteoblasts to stimulate new bone growthand improve bone density.
Biological Activity
parathyroid hormone (1-34) (human), (c181h291n55o51s2), a peptide with the sequence h2n-svseiqlmhnlgkhlnsmervewlrkklqdvhnf-oh, mw= 4117.72. parathyroid hormone (pth), parathormone or parathyrin, is secreted by the chief cells of the parathyroid glands as a polypeptide containing 84 amino acids. it acts to increase the concentration of calcium (ca2+) in the blood, whereas calcitonin (a hormone produced by the parafollicular cells (c cells) of the thyroid gland) acts to decrease calcium concentration. pth acts to increase the concentration of calcium in the blood by acting upon the parathyroid hormone 1 receptor and the parathyroid hormone 2 receptor(1). parathyroid hormone regulates serum calcium, it enhances the release of calcium from the large reservoir contained in the bones(2). it enhances active reabsorption of calcium and magnesium from distal tubules and the thick ascending limb(3). it enhances the absorption of calcium in the intestine by increasing the production of activated vitamin d.figure1 formula of parathyroid hormone (1-34) (human)figure2 the parathyroid hormone (pth) system
Biochem/physiol Actions
Parathyroid hormone (PTH) is a parathyroid-secreted polypeptide hormone that increases the level of calcium in blood by enhancing calcium mobilization from bone, increasing the calcium:phosphate ratio in the kidney and promoting the absorption of calcium by the intestines. PTH is an anabolic agent that improves osteoblastic bone development. PTH 1-34 induces bone morphogenetic protein (BMP) gene transcription. Active N-terminal fragment of PTH (residues 1–34), has been used as a therapeutic for postmenopausal women with osteoporosis who are at high risk for fracture.
Pharmacokinetics
If administered once daily or intermittently, teriparatide preferentially enhances osteoblastic function,and bone formation occurs. Continuous exposure to endogenous PTH may result in poor skeletal composition because of enhanced osteoclast-mediated bone resorption. After 18 months of treatment, lumbar BMD increased up to 12% in postmenopausal women. After 10 months of treatment, 53% of men had an increase of 5% or greater in spine BMD. The risk for developing new vertebral fractures was reduced by 65% after 21 months of treatment, and the number of nonvertebral fragility fractures was reduced by 53%.
Clinical Use
In 2002, the U.S. FDA approved teriparatide for the treatment of postmenopausal osteoporosis in patients who have a high risk of fracture as well as to increase bone mass in men with primary or hypogonadal osteoporosis who have a high risk of fracture. Teriparatide is recombinant human PTH 1-34, the biologically active portion of the endogenously produced preprohormone. Unlike the bisphosphonates, which are classified as bone restorative agents, teriparatide is the first approved bone-forming agent. Bone formation is possible because of the ability of this agent to increase the number of osteoblasts. Although teriparatide enhances the function of both osteoclasts and osteoblasts, the exposure incidence dictates its effect on the skeleton.
Side Effects
Temporary increases in serum calcium levels occur following administration of teriparatide. As a result, this agent is contraindicated in patients who are predisposed to hypercalcemia. Some evidence suggests that these elevations in serum calcium levels may cause a patient who is taking digitalis to experience digitalis toxicity (39). Teriparatide should not be prescribed to patients with Paget's disease, children, young adults, women who are pregnant or nursing, and those patients who have received skeletal radiation therapy. Because of an increased incidence of osteosarcoma (malignant bone tumors) observed in rats, teriparatide also carries a black box warning
storage
Store at -20°C
Dosage
Teriparatide acetate requires subcutaneous injection into the thigh or abdominal wall, and the recommended dose is 20 mcg once daily. If symptoms of orthostatic hypotension occur, it is administered while the patient is sitting or lying down. It is not recommended to use the drug for more than 2 years in the lifetime of the patient. Use teriparatide injection with caution in patients receiving digoxin. Transient hypercalcemia may predispose patients to digitalis toxicity.
Properties of Teriparatide acetate
| Melting point: | >205oC (dec.) |
| RTECS | SQ7770000 |
| storage temp. | −20°C |
| solubility | DMSO (Slightly), Water (Slightly) |
| form | powder |
| color | White to Off-White |
| Water Solubility | Soluble to 0.40 mg/ml in water |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 52232-67-4(CAS DataBase Reference) |
Safety information for Teriparatide acetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H227:Flammable liquids H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. P501:Dispose of contents/container to..… |
Computed Descriptors for Teriparatide acetate
Teriparatide acetate manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
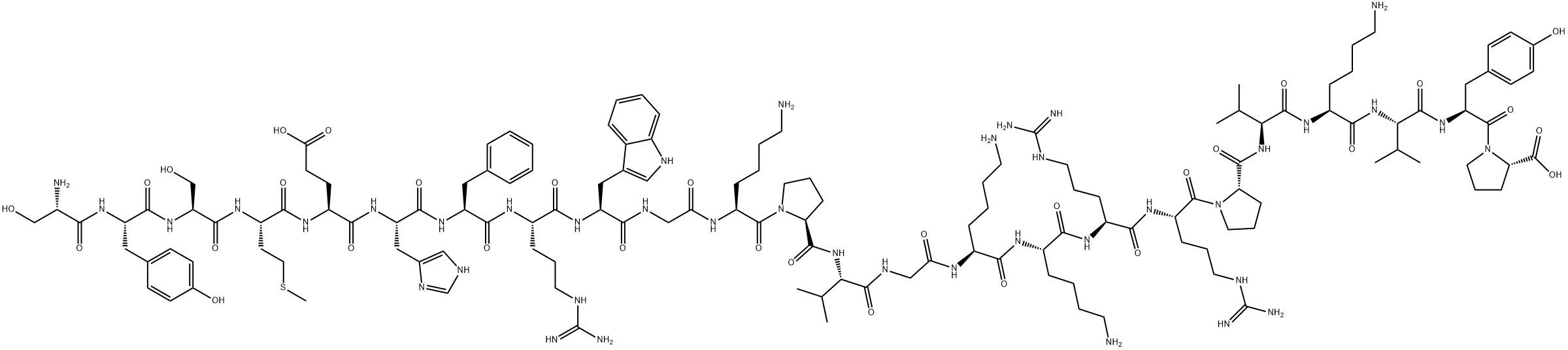


You may like
-
 Parathyroid Hormone 1-34, Human CAS 52232-67-4View Details
Parathyroid Hormone 1-34, Human CAS 52232-67-4View Details
52232-67-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8