Lamivudine
Synonym(s):Lamivudine;Epivir;Epivir-HBV;Heptovir;Zeffix
- CAS NO.:134678-17-4
- Empirical Formula: C8H11N3O3S
- Molecular Weight: 229.26
- MDL number: MFCD00869739
- EINECS: 603-844-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
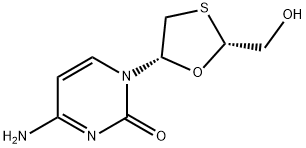
What is Lamivudine?
Absorption
Lamivudine was rapidly absorbed after oral administration in HIV-infected patients. Absolute bioavailability in 12 adult patients was 86% ± 16% (mean ± SD) for the 150-mg tablet and 87% ± 13% for the oral solution. The peak serum lamivudine concentration (Cmax) was 1.5 ± 0.5 mcg/mL when an oral dose of 2 mg/kg twice a day was given to HIV-1 patients. When given with food, absorption is slower, compared to the fasted state.
Toxicity
The most common reported adverse reactions (incidence ≥15%) in adults were headache, nausea, malaise and fatigue, nasal signs and symptoms, diarrhea, and cough.
Description
Lamivudine is a new generation orally active nucleoside analog launched in the U.S.A. for use in combination with zidovudine (AZT) as a first-line therapy for patients with HIV infection. Lamivudine is rapidly converted to phosphorylated metabolites in the body which act as inhibitors and chain terminators of HIV reverse transcriptase (RT), the enzyme required for the replication of the HIV genome. Lamivudine has similar inhibitory potency to RT as AZT but is 10 times less toxic and is active against AZT-resistant strains of HIV.
Chemical properties
White solid from methanol-ethyl acetate. [α]D21-132° (C=1.08, methanol). Or crystallised from boiling ethanol, melting point 160-162°C. [α]D21-135° (C=0.38, methanol).
Originator
BioChem Pharma (Canada)
History
Lamivudine[134678-17-4] is produced by GlaxoSmithKline LLC. In the early 1990s, it was used by some countries in Europe and North America to treat AIDS. In the mid-1990s, medical experts found that it had an inhibitory effect on the DNA of hepatitis B virus. In 1998, the US Food and Drug Administration (FDA) first approved it as a treatment drug for hepatitis B. In 1999, the China Food and Drug Administration approved this drug as a hepatitis B treatment drug, with the Chinese trade name "Heputin".
The Uses of Lamivudine
Lamivudine is used along with other medications to treat human immunodeficiency virus (HIV) infection in adults and children 3 months of age and older.
Lamivudine (Epivir-HBV) is used to treat hepatitis B infection.
Lamivudine is in a class of medications called nucleoside reverse transcriptase inhibitors (NRTIs). It works by decreasing the amount of HIV and hepatitis B in the blood.
Indications
For the treatment of HIV infection and chronic hepatitis B (HBV).
Background
A reverse transcriptase inhibitor and zalcitabine analog in which a sulfur atom replaces the 3' carbon of the pentose ring. It is used to treat Human Immunodeficiency Virus Type 1 (HIV-1) and hepatitis B (HBV).
What are the applications of Application
Lamivudine is a potent reverse transcriptase inhibitor
Definition
ChEBI: Lamivudine is a monothioacetal that consists of cytosine having a (2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl moiety attached at position 1. An inhibitor of HIV-1 reverse transcriptase.
Synthesis
Aqueous hydrochloric acid (6N, 30 ml) was slowly added to a solution of 20 gm of the solid (2R-cis)-4-Amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidi- none.S-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate in water (200 ml) at 45-50 deg C. Stirred the reaction for 1 hour at room temperature. The solid S-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate was filtered, and the aqueous layer was neutralized with an aqueous sodium hydroxide solution (30%, 20 ml). The solvent was recovered under vacuum at 40-45 ℃, the product obtained was dissolved in methanol (200 ml), filtered to remove the inorganic salts, the filtrate was concentrated under vacuum at 40-45 ℃, and the residual solid obtained was dissolved in ethanol (50 ml), heated to 50 ℃, slowly allowed to room temperature, cooled to 10 ℃, filtered and dried at 40-45 ℃. to obtain 5 gm of Lamivudine.
brand name
Epivir (GlaxoSmithKline).
Acquired resistance
Long-term lamivudine administration frequently elicits viral resistance characterized by a reincrease of viral replication in an adherent patient. The incidence of lamivudine resistance is 14% to 32% after 1 year of treatment, 38% after 2 years, and 53% to 76% after 3 years.
Pharmacokinetics
The pharmacokinetics of lamivudine are similar in patients with HIV-1 or HBV infection, and healthy volunteers. The drug is rapidly absorbed after oral administration, with maximum serum concentrations usually attained 0.5 to 1.5 hours after the dose.
Clinical Use
Lamivudine is indicated for the treatment of HIV when used in combination with other antiretroviral agents.A lower dose than that used to treat HIV is approved for the treatment of HBV.
Side Effects
Lamivudine oral tablet can cause mild or serious side effects. The more common side effects that can occur with lamivudine include:
cough,diarrhea,fatigue,headache,malaise (general discomfort),nasal symptoms, such as a runny nose,nausea.
Drug interactions
There are potentially dangerous interactions with other drugs when used in combination.
Antimicrobials: trimethoprim inhibits the excretion of lamivudine.
Antivirals: avoid concomitant use with foscarnet, emtricitabine, and IV ganciclovir
Cytotoxic drugs: avoid concomitant use with clarithromycin.
Orlistat: absorption may be reduced by orlistat.
Biologiacal activity
Lamivudine is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1) and hepatitis B (HBV) to disrupt viral DNA synthesis. When phosphorylated, lamivudine can form active metabolites that compete for incorporation into viral DNA. Via DNA incorporation, lamivudine metabolites competitively inhibit the activity of the HIV reverse transcriptase enzyme and act as a chain terminator of DNA synthesis. Due to the lack of a 3'-OH group, incorporated nucleoside analogues prevent forming a 5' to 3' phosphodiester linkage essential for DNA chain elongation.
Metabolism
Metabolism of lamivudine is a minor route of elimination. In man, the only known metabolite of lamivudine is the trans-sulfoxide metabolite. This biotransformation is catalyzed by sulfotransferases. Lamivudine is metabolized intracellularly to the active antiviral triphosphate. Hepatic metabolism is low (5-10%), and the majority of lamivudine is excreted unchanged in the urine via glomerular filtration and active secretion (organic cationic transport system).
References
[1] ROUSSOSA. Lamivudine treatment for acute severe hepatitis B: report of a case and review of the literature.[J]. Acta gastro-enterologica Belgica, 2008, 71 1: 30-32.
[2] ARTSE J WainbergM A. Mechanisms of nucleoside analog antiviral activity and resistance during human immunodeficiency virus reverse transcription.[J]. Antimicrobial Agents and Chemotherapy, 1996, 40 3: 527-540. DOI:10.1128/AAC.40.3.527.
[3] COATESJ A. (-)-2’-deoxy-3’-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro.[J]. Antimicrobial Agents and Chemotherapy, 1992, 36 4: 733-739. DOI:10.1128/AAC.36.4.733.
[4] KONGHUI. Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy.[J]. Cell Death & Disease, 2022, 13 4: 336. DOI:10.1038/s41419-022-04786-w.
[5] KUMAR P N, PATEL P. Lamivudine for the treatment of HIV[J]. Expert Opinion on Drug Metabolism & Toxicology, 2010, 6: 105-114. DOI:10.1517/17425250903490418.
[6] PERRYC M FauldsD. Lamivudine. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection.[J]. Drugs, 1997, 53 4: 657-680. DOI:10.2165/00003495-199753040-00008.
[7] RAJURKARMIHIR. Reverse Transcriptase Inhibition Disrupts Repeat Element Life Cycle in Colorectal Cancer.[J]. Cancer discovery, 2022, 12 6: 1462-1481. DOI:10.1158/2159-8290.CD-21-1117.
Properties of Lamivudine
| Melting point: | 177 °C |
| Boiling point: | 475.4±55.0 °C(Predicted) |
| alpha | D21 -135° (c = 0.38 in methanol) |
| Density | 1.73±0.1 g/cm3(Predicted) |
| refractive index | -142 ° (C=1, MeOH) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | water: soluble10mg/mL, clear |
| pka | 13.83±0.10(Predicted) |
| form | powder |
| color | white to beige |
| Water Solubility | 70g/L(temperature not stated) |
| Merck | 14,5352 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 134678-17-4(CAS DataBase Reference) |
| EPA Substance Registry System | 2(1H)-Pyrimidinone, 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]- (134678-17-4) |
Safety information for Lamivudine
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Lamivudine
| InChIKey | JTEGQNOMFQHVDC-NKWVEPMBSA-N |
Lamivudine manufacturer
KRS Pharmaceuticals Pvt. Ltd.
HRV Global Life Sciences
Amara Labs Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 134678-17-4 98%View Details
134678-17-4 98%View Details
134678-17-4 -
 Lamivudine 98%View Details
Lamivudine 98%View Details -
 Lamivudine 98% CAS 134678-17-4View Details
Lamivudine 98% CAS 134678-17-4View Details
134678-17-4 -
 Lamivudine CAS 134678-17-4View Details
Lamivudine CAS 134678-17-4View Details
134678-17-4 -
 Lamivudine CAS 134678-17-4View Details
Lamivudine CAS 134678-17-4View Details
134678-17-4 -
 Lamivudine CAS 134678-17-4View Details
Lamivudine CAS 134678-17-4View Details
134678-17-4 -
 Lamivudine CAS 134678-17-4View Details
Lamivudine CAS 134678-17-4View Details
134678-17-4 -
 Lamivudine Resolution Mixture A CASView Details
Lamivudine Resolution Mixture A CASView Details