Lafutidine
- CAS NO.:118288-08-7
- Empirical Formula: C22H29N3O4S
- Molecular Weight: 431.55
- MDL number: MFCD00867520
- EINECS: 601-513-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
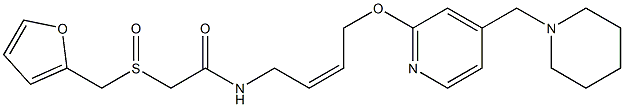
What is Lafutidine?
Description
Lafutidine was launched in Japan for the treatment of gastritis, reflux oesophagitis and peptic ulcers. It can be prepared in eight steps from 4-(2-tetrahydropyranyloxy)-2(Z)-butenl- ol. Lafutidine is a potent and longer-acting H2 antagonist compared to other marketed compounds of its class such as cimetidine and famotidine. In contrast to other commercially available H2 antagonists, lafutidine also exerts a gastroprotective action probably via capsaicin-sensitive afferent nerves. It was clinically effective in the treatment of nonsteroidal antiinflammatory drug-induced ulcer in patients refractory to existing antiulcer agents.
Description
Lafutidine is a histamine H2 receptor antagonist with gastroprotective activity. It inhibits histamine-induced cAMP production in CHO cells expressing human histamine H2 receptors when used at a concentration of 10 nM. Intragastric administration of lafutidine (3, 10, and 30 mg/kg) reduces hemorrhagic esophageal lesion size and gastric acid secretion in a rat model of pyloric ligation-induced reflux esophagitis. It prevents 5-fluorouracil-induced intestinal mucositis, diarrhea, and body weight loss in wild-type, but not Trpv1-/- or sensory deafferented, mice when administered at doses ranging from 3 to 30 mg/kg. Lafutidine (10 mg/kg) also reduces indomethacin-induced antral ulcer size in wild-type, but not chemically-deafferented, rats.
Originator
Fujirebio (Japan)
The Uses of Lafutidine
Lafutidine, a newly developed histamine H(2)-receptor antagonist, inhibits gastric acid secretion
The Uses of Lafutidine
Second generation histamine H2-receptor antagonist. Antiulcerative
The Uses of Lafutidine
(Z)-2-((Furan-2-ylmethyl)sulfinyl)-n-(4-((3-(piperidin-1-ylmethyl)pyridin-2-yl)oxy)but-2-en-1-yl)acetamide is a Histaminic H2 receptor antagonists in ulcer disease. Also, it is a model compound used to investigate the binding mechanism between antiulcer drugs and human serum albumin (HSA).
Definition
ChEBI: Lafutidine is an organic molecular entity.
brand name
Stogar, Protecadin
Properties of Lafutidine
| Melting point: | 92.7-94.9° |
| Boiling point: | 704.2±60.0 °C(Predicted) |
| Density | 1.252±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| Water Solubility | Insoluble in water |
| solubility | DMF:5.0(Max Conc. mg/mL);11.59(Max Conc. mM) DMSO:48.67(Max Conc. mg/mL);112.77(Max Conc. mM) DMSO:PBS (pH 7.2) (1:10):0.09(Max Conc. mg/mL);0.21(Max Conc. mM) Ethanol:9.0(Max Conc. mg/mL);20.86(Max Conc. mM) |
| pka | 13.13±0.46(Predicted) |
| form | powder to crystal |
| color | White to Orange to Green |
| CAS DataBase Reference | 118288-08-7(CAS DataBase Reference) |
Safety information for Lafutidine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lafutidine
Lafutidine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



![4-[4-(Piperidinomethyl)pyridyl-2-oxy]-cis-2-butenamine](https://img.chemicalbook.in/CAS/GIF/118288-25-8.gif)
You may like
-
 118288-08-7 98%View Details
118288-08-7 98%View Details
118288-08-7 -
 Lafutidine, ≥98% (HPLC) CAS 118288-08-7View Details
Lafutidine, ≥98% (HPLC) CAS 118288-08-7View Details
118288-08-7 -
 Lafutidine CAS 118288-08-7View Details
Lafutidine CAS 118288-08-7View Details
118288-08-7 -
 Lafutidine CAS 118288-08-7View Details
Lafutidine CAS 118288-08-7View Details
118288-08-7 -
 Lafutidine API, Grade Standard: Bio-Tech Grade, 118288-08-7View Details
Lafutidine API, Grade Standard: Bio-Tech Grade, 118288-08-7View Details
118288-08-7 -
 99 % Lafutidine API Powder, 118288-08-7View Details
99 % Lafutidine API Powder, 118288-08-7View Details
118288-08-7 -
 99.99 Powder Lafutidine API, 118288-08-7View Details
99.99 Powder Lafutidine API, 118288-08-7View Details
118288-08-7 -
 Lafutidine Powder APIView Details
Lafutidine Powder APIView Details
118288-08-7
