LACTACYSTIN
- CAS NO.:133343-34-7
- Empirical Formula: C15H24N2O7S
- Molecular Weight: 376.43
- MDL number: MFCD01076525
- EINECS: 200-258-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-21 14:56:34
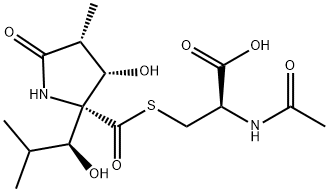
What is LACTACYSTIN?
Description
Lactacystin is a microbial metabolite isolated from Streptomyces that is now widely used as a selective inhibitor of the 20S proteasome. Lactacystin was first characterized by its ability to induce differentiation and inhibit cell cycle progression in several tumor cell lines. At concentrations from 2 to 10 μM, lactacystin induces the outgrowth of neurites in the neuroblastoma cell line Neuro2a. Lactacystin irreversibly alkylates subunit X of the 20S proteasome. The concomitant inhibition of proteasome peptidase activity results in the accumulation of a variety of ubiquitinated proteins which would normally undergo rapid degradation. Thus, the effects of lactacystin are pleiotropic and depend substantially on the expression pattern of signalling proteins within the treated cell.
Chemical properties
White Powder
The Uses of LACTACYSTIN
Lactacystin has been used:
- as a proteasome inhibitor to inhibit protein degradation
- to inhibit proteasomal activity of cells for live cell imaging
- to block proteasomal proteolysis in human monocyte-derived dendritic cells (MoDCs) for 24 h
- to provide unilateral injection to animals to induce nigrostriatal lesions
The Uses of LACTACYSTIN
A selective and potent inhibitor of proteasome-mediated degradation of ubiquitin-tagged proteins. A Streptomyces metabolite that acts as a highly specific inhibitor of the 20S proteasome (MCP: multicatalytic proteinase complex)
What are the applications of Application
Lactacystin is a proteasome inhibitor and cathepsin A inhibitor.
Definition
ChEBI: L-Cysteine substituted at nitrogen by an acetyl group and at sulfur by a substituted-lactam carbonyl group.
General Description
Lactacystin is an antibiotic?and a metabolite of Streptomyces?spp.
Biochem/physiol Actions
Lactacystin can block the development of cell cycle and stimulate differentiation in a murine neuroblastoma cell line. It can serve as a precursor for?clasto-lactacystin β-lactone. Cell-permeable and irreversible proteasome inhibitor (Ki = 4nM). Inhibits NF-kB activation (IC50 = 10mM). Induces neurite outgrowth in neuro2A mouse neuroblastoma cells.
Storage
+4°C
References
1) Omura et al. (1991), Lactacystin, a novel microbial metabolite, induces neuritogenesis of neuroblastoma cells; J. Antibiot., 44 113 2) Fenteany et al. (1995), Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin; Science, 268 726
Properties of LACTACYSTIN
| Melting point: | 233-235°C dec. |
| Boiling point: | 714.9±60.0 °C(Predicted) |
| Density | 1.367±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 20 mg/ml), in Water (up to 10 mg/ml), or in Ethanol (up to 1 mg/ml). |
| form | powder |
| pka | 3.11±0.10(Predicted) |
| color | White to off-white |
| Water Solubility | Soluble to 10 mM in water |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. Solutions in water are not stable and must be used with one working day. Aqueous solutions should not be stored. |
Safety information for LACTACYSTIN
Computed Descriptors for LACTACYSTIN
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Lactacystin CAS 133343-34-7View Details
Lactacystin CAS 133343-34-7View Details
133343-34-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
