L-Asparagine
Synonym(s):(S)-2-Aminosuccinic acid 4-amide;L -Aspartic acid 4-amide
- CAS NO.:70-47-3
- Empirical Formula: C4H8N2O3
- Molecular Weight: 132.12
- MDL number: MFCD00064401
- EINECS: 200-735-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
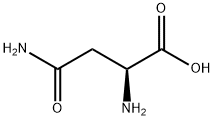
What is L-Asparagine?
Description
Asparagine (abbreviated as Asn or N) is one of the 20 most common natural amino acids on Earth. It has carboxamide as the sidechain's functional group. It is not an essential amino acid. Its codons are AAU and AAC.
The amino acid L-asparagine is a structural analog of L-aspartic acid, where the side chain of the carboxylic acid moiety is amidated, to give a terminal amine group. This renders L-asparagine neutral at physiological pH. The amide group of asparagine is derived from glutamate, in the reaction of aspartate and glutamine in the presence of ATP to yield asparagine and glutamate. In vivo, asparagine is hydrolyzed to aspartate and NH4+ by asparaginase. Asparagine is also an important amino acid in glycopeptide bonds, via N-glycosyl linkages to the sugar rings.
Chemical properties
White crystal or crystalline powder with a slightly sweet taste. Slightly soluble in water, insoluble in ethanol and ether, it often exists as a monohydrate, and it is a rhombic hemihedral crystals. The melting point is 234-235°C , and the aminocarbonyl reaction is carried out by co-heating with sugar, which can form special aroma substances. The best recrystallization method is water, followed by ethanol. In case of alkali hydrolysis into aspartic acid. Heating its aqueous solution also decomposes. Natural products exist in various legumes and the like.
Physical properties
Solubility 3.11 (28 ℃) g/100 g H2O, pI 5.41, dissociation constants: pK1 2.02, pK2 8.8.
Occurrence
Dietary sources
Asparagine is not essential for humans, which means that it can be synthesized from central metabolic pathway intermediates and is not required in the diet. Asparagine is found in :
Animal sources : dairy, whey, beef, poultry, eggs, fish, lactalbumin , sea food
Plant sources : asparagus, potatoes, legumes, nuts, seeds, soy, whole grains.
Biosynthesis
The precursor to asparagine is oxaloacetate. Oxaloacetate is converted to aspartate using a transaminase enzyme. The enzyme transfers the amino group from glutamate to oxaloacetate producing α- ketoglutarate and aspartate. The enzyme asparagine synthetase produces asparagine, AMP, glutamate, and pyrophosphate from aspartate, glutamine, and ATP. In the asparagine synthetase reaction, ATP is used to activate aspartate, forming β-aspartyl-AMP. Glutamine donates an ammonium group, which reacts with β-aspartyl-AMP to form asparagine and free AMP.
History
Asparagine was first isolated in 1806, under a crystalline form, by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet (then a young assistant) from asparagus juice, in which it is abundant — hence, the name they chose for that new matter — becoming the first amino acid to be isolated.
A few years later, in 1809, Pierre Jean Robiquet again identified, this time from liquorice root, a substance with properties he qualified as very similar to those of asparagine, that Plisson in 1828 identified as asparagine itself.
The Uses of L-Asparagine
L-asparagine has been used:
to identify and quantify free amino acids released upon oxidation of proteins and peptides by hydroxyl radicals.
to study the effects of amino acids in promoting food consumption in Drosophila melanogaster.
to study non-enzymatic gluconeogenesis.
L-Asparagine is used in cell culture media and is a component of MEM non-essential amino acids solution.
L-Asparagine has been shown to enhance ornithine decarboxylase activity in cultured human colon adenocarcinoma Caco-2 cells and in cultured IEC-6 intestinal epithelial cells. Spore germination in Bacillus subtilis has been increased in the presence of L-asparagine.
An isoxazoline RGD mimic platelet GPIIb/IIIa antagonist has been prepared by chiral synthesis with L-asparagine as a starting material. L-Asparagine has been utilized in the synthesis of 4-azalysine building blocks for application to combinatorial chemistry.
Background
A non-essential amino acid that is involved in the metabolic control of cell functions in nerve and brain tissue. It is biosynthesized from aspartic acid and ammonia by asparagine synthetase. (From Concise Encyclopedia Biochemistry and Molecular Biology, 3rd ed)
Indications
Used for nutritional supplementation, also for treating dietary shortage or imbalance.
Definition
ChEBI: L-asparagine is an optically active form of asparagine having L-configuration. It has a role as a nutraceutical, a micronutrient, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a plant metabolite. It is an aspartate family amino acid, a proteinogenic amino acid, an asparagine and a L-alpha-amino acid. It is a conjugate base of a L-asparaginium. It is a conjugate acid of a L-asparaginate. It is an enantiomer of a D-asparagine. It is a tautomer of a L-asparagine zwitterion.
Production Methods
A simple synthesis of L -asparagine starts from L -aspartic acid which is esterified to the b-methyl ester followed by treatment with ammonia.
Biological Functions
Asparagine is a dietarily dispensable amino acid synthesized from aspartate and glutamine. Asparagine has three major functions: 1) incorporation into amino acid sequences of proteins; 2) storage form for aspartate (is a required precursor for synthesis of DNA, RNA and ATP); and 3) source of amino groups for production of other dispensable amino acids via trasaminases.
The nervous system requires asparagine. It also plays an important role in the synthesis of ammonia.
The addition of N-acetyl glucosamine to asparagine is performed by oligosaccharyltransferase enzymes in the endoplasmic reticulum. This glycosylation is important both for protein structure and protein function.
Biochem/physiol Actions
L-asparagine is an uncharged derivative of aspartate. It possesses a polar side chain and is a non-essential amino acid.
Pharmacokinetics
A non-essential amino acid. Asparagine is critical for the production of the body's proteins, enzymes and muscle tissue. Supplements of this amino acid are claimed to balance nervous system function.
Safety Profile
When heated to decomposition emits toxic fumes of Nox
Metabolism
Not Available
Purification Methods
Likely impurities are aspartic acid and tyrosine. Crystallise it from H2O or aqueous EtOH. It slowly effloresces in dry air. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 1856 1961, Beilstein 4 IV 3005.]
Degradation
Aspartate is a glucogenic amino acid. L-asparaginase hydrolyzes the amide group to form aspartate and ammonium. A transaminase converts the aspartate to oxaloacetate, which can then be metabolized in the citric acid cycle or gluconeogenesis.
Properties of L-Asparagine
| Melting point: | 235 °C (dec.) (lit.) |
| Boiling point: | 244.01°C (rough estimate) |
| alpha | 34.5 º (c=10, 2N HCl) |
| Density | 1,543g/cm |
| refractive index | 1.4880 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Practically insoluble in methanol, ethanol, ether, benzene. Soluble in acids and alkalies. |
| form | Powder |
| pka | 2.17(at 20℃) |
| color | White |
| Odor | sltly sweet taste |
| Water Solubility | H2O: 20 g/L (20 oC) , clear, colorless |
| Sensitive | Hygroscopic |
| Merck | 14,837 |
| BRN | 1723527 |
| Stability: | Stable, but may be moisture-sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 70-47-3(CAS DataBase Reference) |
| NIST Chemistry Reference | L-Asparagine(70-47-3) |
| EPA Substance Registry System | L-Asparagine (70-47-3) |
Safety information for L-Asparagine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for L-Asparagine
L-Asparagine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
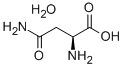



You may like
-
 L-Asparagine 99%View Details
L-Asparagine 99%View Details -
 L-Asparagine 95% CAS 70-47-3View Details
L-Asparagine 95% CAS 70-47-3View Details
70-47-3 -
 Asparagine anhydrous CAS 70-47-3View Details
Asparagine anhydrous CAS 70-47-3View Details
70-47-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
