Isoprenaline hydrochloride
Synonym(s):(±)-Isoproterenol hydrochloride;N-Isopropyl-DL -noradrenaline hydrochloride;1-(3′,4′-Dihydroxyphenyl)-2-isopropylaminoethanol hydrochloride;Isoprenaline hydrochloride;Isoproterenol, Hydrochloride - CAS 51-30-9 - Calbiochem
- CAS NO.:51-30-9
- Empirical Formula: C11H18ClNO3
- Molecular Weight: 247.72
- MDL number: MFCD00012603
- EINECS: 200-089-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
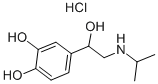
What is Isoprenaline hydrochloride ?
Description
Isoprenaline hydrochloride is an FDA-approved injectable drug that is the hydrochloride form of isoprenaline and is used as a bronchodilator, adrenergic beta agonist, cardiotonic, and sympathomimetic. Selective beta-adrenergic agonist that increases cytoplasmic cAMP. promotes glycogenolysis and causes bronchodilation. Increases Cl- diffusion and potential difference in rabbit tracheal cells via nitric oxide production. In addition, it can be used as an inducer in an experimental rat model of induced myocardial infarction[1].
Chemical properties
White or almost white, crystalline powder.
The Uses of Isoprenaline hydrochloride
Isoproterenol is a non-selective beta-adrenergic agonist. Isoproterenol is used in the treatment of bradycardia; bronchodilator.
The Uses of Isoprenaline hydrochloride
Isoproterenol hydrochloride is used as a selective β-adrenergic agonist, increases cytosolic cAMP.
What are the applications of Application
Isoproterenol Hydrochloride is a selective β-adrenergic agonist
Definition
ChEBI: DL-Isoprenaline hydrochloride is a member of catechols.
brand name
Isuprel (Hospira); Isuprel (Sanofi Aventis); Vapo- ISO (Fisons).
General Description
Odorless white crystalline powder. Slightly bitter taste. Aqueous solutions turn brownish-pink upon prolonged exposure to air.
Air & Water Reactions
May be sensitive to exposure to air and light. . Water soluble.
Reactivity Profile
An acid organic salt. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
SYMPTOMS: Symptoms of exposure to Isoprenaline hydrochloride may include tremors, nervous apprehension, palpitations of the heart, epigastric distress, cardiac arrhythmias, myocardial necrosis, cardiac arrest, tachycardia, headache, flushing of the skin, anginal pain, nausea, dizziness, weakness, and sweating.
Fire Hazard
Flash point data for Isoprenaline hydrochloride are not available. Isoprenaline hydrochloride is probably combustible.
Biological Activity
Standard selective β -adrenoceptor agonist; vasorelaxant and bronchodilator. Activation of β 2 receptors activates downstream PKA and ERK, and may stimulate NO-mediated endothelium-dependent smooth muscle relaxation. Active in vivo . Also available as part of the β -Adrenoceptor Agonist Tocriset™ and Mixed Adrenergic Tocriset™ .
Biochem/physiol Actions
β-adrenoceptor agonist; increases cytosolic cAMP.
storage
Store at RT
References
[1] FANG WANG. Fucoxanthin averts isoprenaline hydrochloride-induced myocardial infarction in rats[J]. Pharmacognosy Magazine, 2020, 16 1: 214-220. DOI:10.4103/pm.pm\_357\_19.
Properties of Isoprenaline hydrochloride
| Melting point: | 165-175 °C (dec.)(lit.) |
| Boiling point: | 192°C (rough estimate) |
| Density | 1.2296 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| solubility | Freely soluble in water, sparingly soluble in ethanol (96 per cent), practically insoluble in methylene chloride. |
| form | White solid |
| color | White to Off-White |
| Water Solubility | Soluble |
| BRN | 3917363 |
| Stability: | Stable, but may be air and light sensitive. Incompatible with strong oxidizing agents. |
| InChI | InChI=1S/C11H17NO3.ClH/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8;/h3-5,7,11-15H,6H2,1-2H3;1H |
| CAS DataBase Reference | 51-30-9(CAS DataBase Reference) |
| EPA Substance Registry System | Isoproterenol hydrochloride (51-30-9) |
Safety information for Isoprenaline hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Isoprenaline hydrochloride
| InChIKey | IROWCYIEJAOFOW-UHFFFAOYSA-N |
| SMILES | C1(=CC=C(O)C(O)=C1)C(O)CNC(C)C.Cl |
Isoprenaline hydrochloride manufacturer
Sai Life Sciences Ltd
Gland Pharma Ltd
Biophore India Pharmaceuticals Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Isoproterenol Hydrochloride CAS 51-30-9View Details
Isoproterenol Hydrochloride CAS 51-30-9View Details
51-30-9 -
 Isoprenaline HCl 98% (HPLC) CAS 51-30-9View Details
Isoprenaline HCl 98% (HPLC) CAS 51-30-9View Details
51-30-9 -
 Isoprenaline hydrochloride CAS 51-30-9View Details
Isoprenaline hydrochloride CAS 51-30-9View Details
51-30-9 -
 Isoprenaline hydrochloride CAS 51-30-9View Details
Isoprenaline hydrochloride CAS 51-30-9View Details
51-30-9 -
 Isoprenaline hydrochloride CAS 51-30-9View Details
Isoprenaline hydrochloride CAS 51-30-9View Details
51-30-9 -
 Isoproterenol hydrochloride CAS 51-30-9View Details
Isoproterenol hydrochloride CAS 51-30-9View Details
51-30-9 -
 Isoproterenol Hydrochloride CAS 51-30-9View Details
Isoproterenol Hydrochloride CAS 51-30-9View Details
51-30-9 -
 Isoproterenol, Hydrochloride CAS 51-30-9View Details
Isoproterenol, Hydrochloride CAS 51-30-9View Details
51-30-9