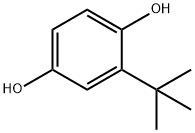
4-tert-Butylcatechol
Synonym(s):4-tert-Butylpyrocatechol
- CAS NO.:98-29-3
- Empirical Formula: C10H14O2
- Molecular Weight: 166.22
- MDL number: MFCD00002201
- EINECS: 202-653-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-23 17:25:30
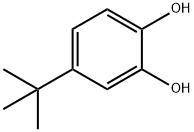
What is 4-tert-Butylcatechol?
Description
Para-tertiary butyl catechol is specially prepared by reacting the impure catechol fraction with tertiary butyl alcohol. Used for its various properties (inhibition of polymerization and as an antioxidizing agent) in the manufacture of rubber, plastics and paints, in the preparation of petrolatum products, and as an antioxidant in oils, it may induce vitiligo.
Chemical properties
white to cream flakes
The Uses of 4-tert-Butylcatechol
Polymerization inhibitor for styrene-butadiene and other olefins.
Inhibitor-remover packings and ready-to-use, disposable prepacked columns offer a quick and convenient means of removing small amounts of inhibitors which are added to reagents or solvents that would otherwise be unstable (e.g., they may polymerize, oxidize or darken) on storage.
The inhibitor removers are useful in applications which require that the stabilizer or inhibitor [i.e., hydroquinone (HQ), hydroquinone monomethyl ether (MEHQ, 4-methoxyphenol) or 4-tert-butylcatechol (TBC)] be removed prior to use.
The Uses of 4-tert-Butylcatechol
4-tert-butylcatechol (PTBC) is used as an antioxidant in fats, oils, and mineral oils, and as a stabilizer in polyester resins and polystyrene resins. A concentration of PTBC (up to 0.005%) can be found in paints, glues, thermal paper, lubricating oil, and mineral oil products.
4-tert-Butylcatechol is widely utilized as an inhibitor in polymerization of butadiene, styrene, vinyl acetate and other reactive monomers. It plays an important role in the synthesis of tungsten oxide nanoparticles by nonaqueous sol-gel process. It acts as a stabilizer in the manufacturing of polyurethane foam. It is employed as an antioxidant for synthetic rubber, polymers and oil derivatives. It is also utilized as a purification agent for aminoformate catalysts.
General Description
4-tert-Butylcatechol,in the presence of O2 catalyzed by tyrosinase, yields 4-tert-butyl-o-benzoquinone. Electrochemical oxidation of 4-tert-Butylcatechol in the presence of 4-hydroxycoumarin as nucleophile has been studied using cyclic voltammetry and controlled-potential coulometry. It undergoes electrochemical trimerization via anodic oxidation and mechanism of trimerization has been studied using cyclic voltammetry and controlled-potential coulometry.
Hazard
Toxic by ingestion and skin absorption.
Flammability and Explosibility
Not classified
Contact allergens
p-tert-Butyl catechol is specially prepared by reacting the impure catechol fraction with tertiary butyl alcohol. It is used for its various properties (inhibitor of polymerization and antioxidizing agent) in the manufacture of rubber, plastics, and paints, in the preparation of petrolatum products, and as an antioxidant in oils. It may induce vitiligo.
Safety Profile
A poison by intravenous route. Moderately toxic by ingestion and skin absorption. A severe skin and eye irritant. Mutation data reported. Combustible when exposed to heat or flame. To fight fire, use Con, dry chemical, fog, mist. When heated to decomposition it emits acrid and irritating fumes
Purification Methods
Distil it in a vacuum, then recrystallise it from pentane or pet ether (or *C6H6). [Beilstein
Properties of 4-tert-Butylcatechol
| Melting point: | 52-55 °C(lit.) |
| Boiling point: | 285 °C(lit.) |
| Density | 1.049 |
| vapor pressure | <1 hPa (25 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | methanol: soluble1g/10 mL, clear, colorless to slightly yellow |
| pka | 9.92±0.10(Predicted) |
| form | powder to lump |
| color | White to Light yellow to Light red |
| Odor | at 10.00?%?in?dipropylene glycol. phenolic |
| Water Solubility | 0.2 g/100 mL (25 ºC) |
| Sensitive | Hygroscopic |
| BRN | 2043335 |
| Exposure limits | ACGIH: TWA 5 ppm (Skin) NIOSH: TWA 5 ppm(20 mg/m3) |
| CAS DataBase Reference | 98-29-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2-Benzenediol, 4-(1,1-dimethylethyl)-(98-29-3) |
| EPA Substance Registry System | p-tert-Butylcatechol (98-29-3) |
Safety information for 4-tert-Butylcatechol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-tert-Butylcatechol
| InChIKey | XESZUVZBAMCAEJ-UHFFFAOYSA-N |
4-tert-Butylcatechol manufacturer
JSK Chemicals
CEFA CILINAS BIOTICS PVT LTD
Antares Chem Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 4-tert-Butylcatechol 98%View Details
4-tert-Butylcatechol 98%View Details -
 4 Tert Butyl Catechol CASView Details
4 Tert Butyl Catechol CASView Details -
 4-tert-Butylcatechol, 99% CAS 98-29-3View Details
4-tert-Butylcatechol, 99% CAS 98-29-3View Details
98-29-3 -
 p-tert-Butyl Catechol pure CAS 98-29-3View Details
p-tert-Butyl Catechol pure CAS 98-29-3View Details
98-29-3 -
 4 Tert Butyl Catechol (TBC), 99%, 25 kg Bag for Synthetic Rubber IndustryView Details
4 Tert Butyl Catechol (TBC), 99%, 25 kg Bag for Synthetic Rubber IndustryView Details
98-29-3 -
 4 Tert Butyl Catechol Tbc Cas 98 29 3View Details
4 Tert Butyl Catechol Tbc Cas 98 29 3View Details
98-29-3 -
 4-tert-Butyl CatecholView Details
4-tert-Butyl CatecholView Details
98-29-3 -
 4 Tert Butyl Catechol, 500 g Packet, 99%View Details
4 Tert Butyl Catechol, 500 g Packet, 99%View Details
98-29-3
