51-30-9
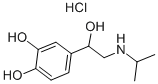
Product Name:
Isoprenaline hydrochloride
Formula:
C11H18ClNO3
Synonyms:
(±)-Isoproterenol hydrochloride;N-Isopropyl-DL -noradrenaline hydrochloride;1-(3′,4′-Dihydroxyphenyl)-2-isopropylaminoethanol hydrochloride;Isoprenaline hydrochloride;Isoproterenol, Hydrochloride - CAS 51-30-9 - Calbiochem
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Isoproterenol hydrochloride is an odorless white crystalline powder. Slightly bitter taste. Aqueous solutions turn brownish-pink upon prolonged exposure to air. (NTP, 1992) |
|---|---|
| Melting Point | 338 to 340 °F (NTP, 1992) |
| Solubility | >37.2 [ug/mL] (The mean of the results at pH 7.4) |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 247.72 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 247.0975211 g/mol |
| Monoisotopic Mass | 247.0975211 g/mol |
| Topological Polar Surface Area | 72.7 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 187 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Isoproterenol hydrochloride is an odorless white crystalline powder. Slightly bitter taste. Aqueous solutions turn brownish-pink upon prolonged exposure to air. (NTP, 1992)
