Isobutyric acid
Synonym(s):2-Methylpropionic acid;Isobutanoic acid;Isobutyric acid
- CAS NO.:79-31-2
- Empirical Formula: C4H8O2
- Molecular Weight: 88.11
- MDL number: MFCD00002658
- EINECS: 201-195-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:39
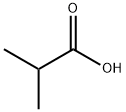
What is Isobutyric acid?
Chemical properties
Isobutyric acid is a clear colorless oily liquid with an odor and flavor similar to n-butyric acid. Miscible with water, soluble in ethanol and ether. Prepared via oxidation of isobutyl alcohol.
Physical properties
Isobutyric Acid is a flavoring agent that is a colorless liquid with a strong, penetrating odor, resembling butter. it is miscible in alcohol, propylene glycol, glycerin, mineral oil, and most fixed oils and soluble in water. it is obtained by chemical synthesis. it is also termed isopropylformic acid.
Occurrence
Isobutyric acid occurs naturally in Ceratonia siliqua L. The gum obtained from the kernels of this species is used as a thickener in the food industry. Reported found in several essential oils: Arnica montana, Roman chamomile, Laurus nobilis, imperatoria, and in carob fruits (Siliqua dulcis); also identified in the essence of Seseli tortuosum, Artemisia transiliensis.
The Uses of Isobutyric acid
Isobutyric acid is used to prepare esters for flavors and perfumes. It is also used as a disinfecting agent, preservative and tanning agent. It finds applications in textile, varnish and the leather industry. Further, it is used as a lactation stimulant in dairy cattle. In addition to this, it is used in the preparation of tetramethylsuccinic acid and diisopropyl ketone.
Definition
ChEBI: Isobutyric acid is a branched fatty acid comprising propanoic acid carrying a methyl branch at C-2. It has a role as a volatile oil component, a plant metabolite and a Daphnia magna metabolite. It is a branched-chain saturated fatty acid, a methyl-branched fatty acid and a fatty acid 4:0. It is a conjugate acid of an isobutyrate.
Preparation
Isobutyric acid is prepared in a similar way to butyric acid, mainly by direct oxidation of isobutanol and isobutyraldehyde, which is obtained by a direct oxidation reaction with air or oxygen.
What are the applications of Application
Isobutyric acid is mainly used in the synthesis of isobutyric acid esters, such as methyl isobutyrate, propyl ester, isoamyl ester and benzyl ester. It can also be used manufacture of esters for solvents, flavors and perfume bases, disinfecting agent, varnish, plasticizers, deliming hides, tanning agent and used in pharmaceutical. Isobutyric acid has some important derivatives that, in the industry, is actually used for the production of isobutyronitrile intermediates, and then converted to isobutylamidine hydrochloride that is the raw materials of pesticide diazinon.
Aroma threshold values
Detection: 10 ppb to 9.5 ppm; aroma characteristics at 10 ppm: acidic pungent, dairy buttery and cheesy with fruity undertones.
Taste threshold values
Taste characteristics at 15 ppm: acidic, sour dairy, creamy, cheese, cultured dairy nuance.
General Description
Isobutyric acid appears as a colorless liquid with a light odor of rancid butter. Flash point 132°F. Density 7.9 lb / gal. Corrosive to metals and tissue.
Air & Water Reactions
Flammable. Water soluble
Reactivity Profile
Isobutyric acid corrodes aluminum and other metals. Flammable hydrogen gas may accumulate in enclosed spaces in which this reaction has taken place [USCG, 1999].
Hazard
Toxic by ingestion, strong irritant to tissue.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion causes irritation of mouth and stomach. Contact with eyes or skin causes irritation.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Biochem/physiol Actions
Odor at 10 ppm
Synthesis
The preparation of isobutyric acid is similar with butyric acid, which is performed by the direct oxidation of isobutyl alcohol and isobutyraldehyde. Isobutyric acid can be directly generated from the oxidation of isobutyraldehyde in air or oxygen. Other manufacturing methods have isobutyronitrile hydrolysis and methacrylic acid hydrogenation. The oxidation of 2-methyl-1-nitropropane to prepare isobutyric acid can also obtain a higher yield. The purification of Isobutyric acid can be realized by azeotropic distillation with water, and anhydrous isobutyric acid can be obtained by the extractive distillation from carbon tetrachloride. Propylene and formic acid ester can react at 50 °C with the catalysis of hydrofluoric acid to generate methyl isobutyrate and propyl isobutyrate.
Purification Methods
Distil the acid from KMnO4, then redistil it from P2O5. [Beilstein 2 H 288, 2 I 126, 2 II 257, 2 III 637, 2 IV 843.]
Properties of Isobutyric acid
| Melting point: | -47 °C (lit.) |
| Boiling point: | 153-154 °C (lit.) |
| Density | 0.95 g/mL at 25 °C (lit.) |
| vapor density | 3.04 (vs air) |
| vapor pressure | 1.5 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2222 | ISOBUTYRIC ACID |
| Flash point: | 132 °F |
| storage temp. | Store below +30°C. |
| solubility | 618g/l |
| form | Liquid |
| pka | 4.84(at 20℃) |
| color | Clear colorless |
| PH | 3.96(1 mM solution);3.44(10 mM solution);2.93(100 mM solution); |
| Odor | at 1.00 % in propylene glycol. acidic sour cheese dairy buttery rancid |
| Odor Threshold | 0.0015ppm |
| explosive limit | 1.6-7.3%(V) |
| Water Solubility | 210 g/L (20 ºC) |
| Merck | 14,5155 |
| JECFA Number | 253 |
| BRN | 635770 |
| Dielectric constant | 2.6(Ambient) |
| CAS DataBase Reference | 79-31-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanoic acid, 2-methyl-(79-31-2) |
| EPA Substance Registry System | Isobutyric acid (79-31-2) |
Safety information for Isobutyric acid
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Isobutyric acid
| InChIKey | KQNPFQTWMSNSAP-UHFFFAOYSA-N |
Isobutyric acid manufacturer
Manav Biochem Impex Private Limited
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Isobutyric Acid extrapure CAS 79-31-2View Details
Isobutyric Acid extrapure CAS 79-31-2View Details
79-31-2 -
 Isobutyric acid CAS 79-31-2View Details
Isobutyric acid CAS 79-31-2View Details
79-31-2 -
 Isobutanoic acid 99% (GC) CAS 79-31-2View Details
Isobutanoic acid 99% (GC) CAS 79-31-2View Details
79-31-2 -
 Isobutanoic acid 97% CAS 79-31-2View Details
Isobutanoic acid 97% CAS 79-31-2View Details
79-31-2 -
 Isobutyric Acid CAS 79-31-2View Details
Isobutyric Acid CAS 79-31-2View Details
79-31-2 -
 ISO BUTYRIC ACIDView Details
ISO BUTYRIC ACIDView Details
79-31-2 -
 Isobutyric AcidView Details
Isobutyric AcidView Details
79-31-2 -
 Isobutyric Acid ChemicalView Details
Isobutyric Acid ChemicalView Details
79-31-2
