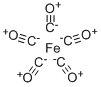Iron carbonyl
- CAS NO.:37220-42-1
- Empirical Formula: Fe(CO)5
- Molecular Weight: 85.87
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
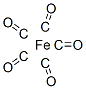
What is Iron carbonyl?
Chemical properties
Mobile, yellow liquid.Evolves carbon monoxide on exposure to air or light. Soluble in nickel tetracarbonyl and most organic solvents; soluble with decomposition in acids and alkalies; insoluble in water.
Chemical properties
Iron pentacarbonyl reacts with cyclopentadiene at 135° in an autoclave to give the
binuclear complex [(π-C5H5)Fe(CO)2]2 as deep reddish-purple crystals which are stable to
air and water. The crystal structure determination shows this molecule to be centrosymmetric with two bridging carbonyls forming a plane with the two iron atoms [Fig. 19(a)]. The rather short Fe-Fe distance of 2-49 ? and the diamagnetism of the compound indicate
the presence of the Fe-Fe bond. In solution, however, it tautomerizes to the structure believed to be that shown in Fig.19(b),in which the two rings lie on one side of the mean
plane formed by the bridging carbonyl groups and the iron atoms and the two terminal CO
groups lie on the other side.
The Uses of Iron carbonyl
Catalyst in organic reactions, carbonyl iron for high-frequency coils.
The Uses of Iron carbonyl
Iron pentacarbonyl is a useful dehalogenating and carbonylating agent.
Production Methods
Although nickel carbonyl can be obtained from nickel and carbon monoxide at atmospheric pressure and moderate temperature, the production of iron pentacarbonyl requires a pressure of 5– 30 MPa, a temperature of 150–200 C, and the presence of reactive iron. Even at high temperature and pressure, massive iron reacts sluggishly with carbon monoxide, so iron sponge, with its greater surface area, is used as starting material.
Hazard
Flammable, dangerous fire risk. Toxic by ingestion, inhalation, and skin absorption.
Chemical Reactivity
Iron pentacarbonyl is an easily combustible substance. It does not react with water or with weak or dilute acids. With concentrated acids, the corresponding iron salts are formed with the evolution of carbon monoxide and hydrogen. Reactions with halogens yield iron halides. Iron pentacarbonyl also reduces organic compounds; for example, nitrobenzene is reduced to aniline; ketones to alcohols; and indigo to indigo white.
Structure and conformation
The trigonal bipyramidal structure has been preferred to the square pyramidal one in the interpretation of electron diffraction, Raman and infrared and thermodynamic data; the apparently contradictory dipole moment of 0-8 D being attributed to atom polarization. A single crystal X-ray structure determination at - 80°C has confirmed the trigonal bipyramidalstructure.
Properties of Iron carbonyl
| Dielectric constant | 2.6(20℃) |
| EPA Substance Registry System | Iron carbonyl (Fe(CO)5), (TB-5-11)-(37220-42-1) |
Safety information for Iron carbonyl
Computed Descriptors for Iron carbonyl
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1