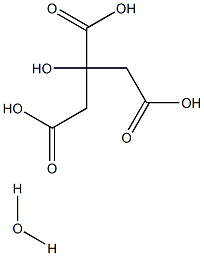
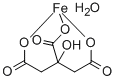
FERRIC CITRATE
Synonym(s):Ferric citrate monohydrate;Iron(III) citrate;Iron(III) citrate hydrate;Iron(III) citrate tribasic monohydrate
- CAS NO.:2338-05-8
- Empirical Formula: C6H8FeO7
- Molecular Weight: 247.97
- MDL number: MFCD00013098
- EINECS: 219-045-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
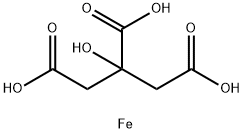
What is FERRIC CITRATE?
Absorption
Ferric iron has been shown to have inferior bioavailability to ferrous iron preparations. Tetraferric tricitrate decahydrate has 19% the bioavailability of ferrous ascorbate.
Toxicity
Patients experiencing an overdose of iron may present with nausea, vomiting, abdominal pain, diarrhea, fluid and blood loss, hypovolemia, hematemesis, perforation, and peritonitis. Mild overdoses can be treated with symptomatic and supportive measures. More severe overdoses may require more intense treatment including chelating agents, and intravenous fluids. Activated charcoal is not expected to be beneficial in the case of iron overdose.
The acute oral LD50 in rats is 1487mg/kg and in mice is 1520mg/kg. The acute dermal LD50 in rabbits is 2000mg/kg.
Chemical properties
red to brown powder
The Uses of FERRIC CITRATE
Ferric Citrate is a nutrient supplement that is prepared from reac- tion of citric acid with ferric hydroxide. it is a compound of indefi- nite ratio of citric acid and iron. the ingredient may be used in infant formula. it is also termed iron (iii) citrate.
The Uses of FERRIC CITRATE
Medicine, blueprint paper.
Indications
Tetraferric tricitrate decahydrate is indicated to control serum phosphorous in adults with chronic kidney disease who require dialysis. Tetraferric tricitrate decahydrate is also indicated to treat iron deficiency anemia in adults with chronic kidney disease who are not on dialysis.
Background
Tetraferric tricitrate decahydrate is an iron containing phosphate binder used to treat hyperphosphatemia and iron deficiency anemia in adults with chronic kidney disease.
Tetraferric tricitrate decahydrate was granted FDA approval on 5 September 2014.
Definition
ChEBI: Iron(III) citrate is an iron chelate resulting from the combination of iron(3+) and citrate(3-). It has a role as an anti-anaemic agent and a nutraceutical. It contains a citrate(3-) and an iron(3+).
Flammability and Explosibility
Not classified
Pharmacokinetics
Tetraferric tricitrate decahydrate is an iron containing product indicated to treat iron deficiency anemia and hyperphosphatemia. It has a wide therapeutic index, as doses can be varied significantly between patients. Tetraferric tricitrate decahydrate has a long duration of action in the treatment of iron deficiency anemia, due to the slow loss of iron from the body, and a moderate duration of action in the treatment of hyperphosphatemia, due to its action being dependant on residence time in the gastrointestinal tract. Patients should be counselled regarding the risk of iron overload.
Safety Profile
When heated to decomposition it emits acrid smoke and irritating fumes.
Metabolism
Ferric cation is converted to ferrous iron by duodenal cytochrome B reductase. The heavy chain ferritin may also convert ferric iron to ferrous iron
Properties of FERRIC CITRATE
| Density | 1.906[at 20℃] |
| vapor pressure | 0Pa at 25℃ |
| form | Solid |
| color | Garnet red powder |
| Odor | Odorless |
| Water Solubility | Soluble in water |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Merck | 13,4050 |
| Stability: | Stable. Light sensitive. |
| CAS DataBase Reference | 2338-05-8(CAS DataBase Reference) |
| EPA Substance Registry System | 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, iron salt (2338-05-8) |
Safety information for FERRIC CITRATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for FERRIC CITRATE
FERRIC CITRATE manufacturer
Lupin Ltd
ShanPar Industries Pvt Ltd
West Bengal Chemical Industries Ltd.
Vasa Pharmachem Pvt Ltd
Global Calcium Pvt Ltd
Biophore India Pharmaceuticals Pvt Ltd
Cleargreens Pharmaceutical Private Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2338-05-8 Iron(III) citrate hydrate 98%View Details
2338-05-8 Iron(III) citrate hydrate 98%View Details
2338-05-8 -
 2338-05-8 98%View Details
2338-05-8 98%View Details
2338-05-8 -
 3522-50-7 / 2338-05-8 Ferric citrate 99%View Details
3522-50-7 / 2338-05-8 Ferric citrate 99%View Details
3522-50-7 / 2338-05-8 -
 Ferric citrate 3522-50-7 / 2338-05-8 98%View Details
Ferric citrate 3522-50-7 / 2338-05-8 98%View Details
3522-50-7 / 2338-05-8 -
 Ferric citrate 99%View Details
Ferric citrate 99%View Details
3522-50-7 / 2338-05-8 -
 3522-50-7 / 2338-05-8 98%View Details
3522-50-7 / 2338-05-8 98%View Details
3522-50-7 / 2338-05-8 -
 Iron(III) citrate tribasic monohydrate CAS 2338-05-8View Details
Iron(III) citrate tribasic monohydrate CAS 2338-05-8View Details
2338-05-8 -
 FERRIC CITRATE 99%View Details
FERRIC CITRATE 99%View Details