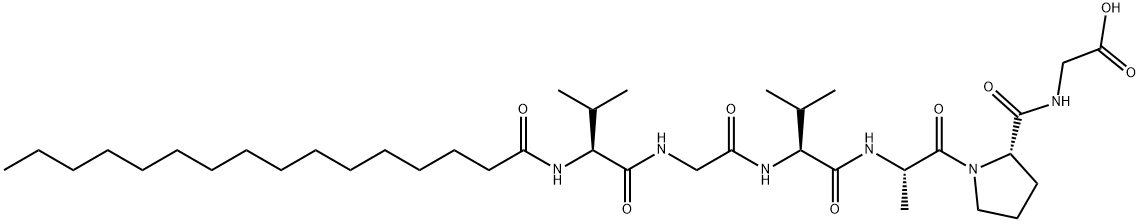
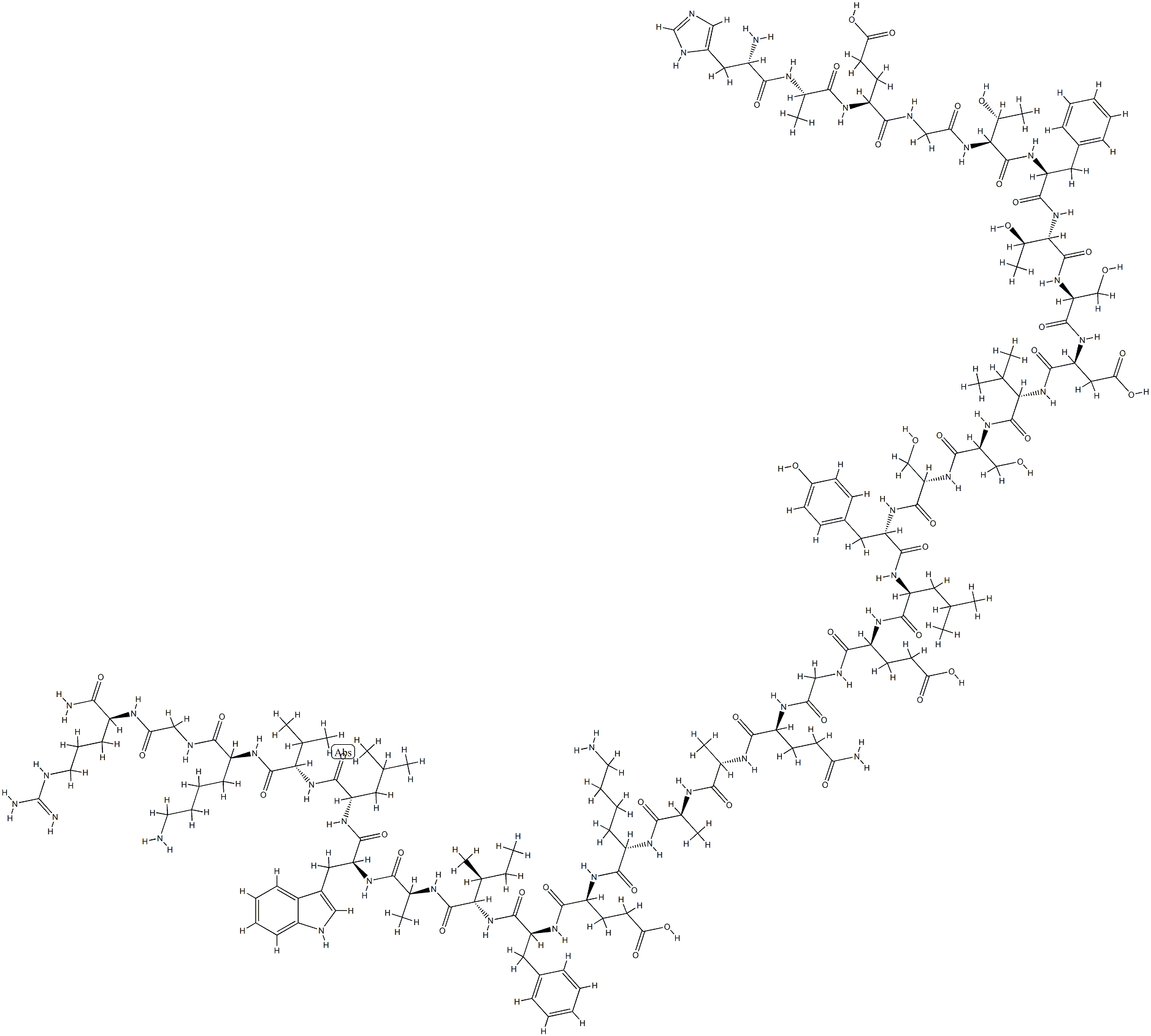
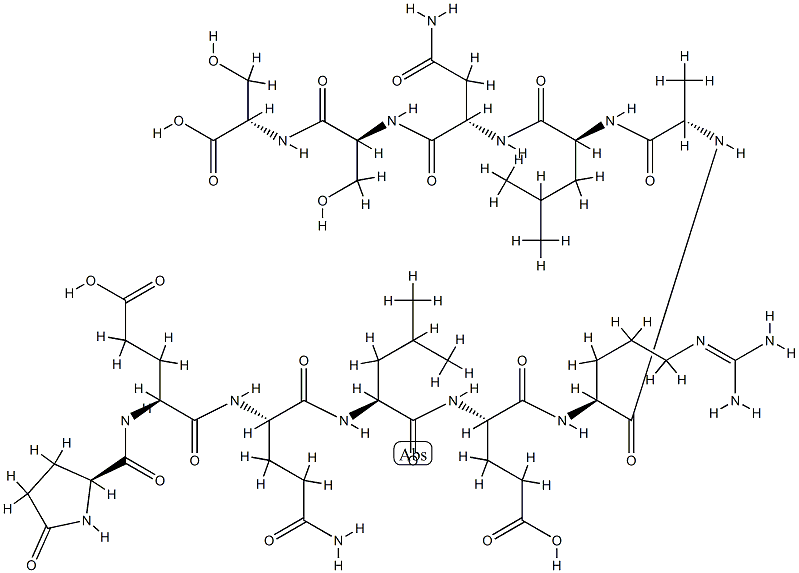
Irofulven
- CAS NO.:920014-72-8
- Empirical Formula: C49H68N18O9S2
- Molecular Weight: 1117.32
- MDL number: MFCD30532719
- EINECS: 000-000-0
- Update Date: 2025-01-01 17:43:08
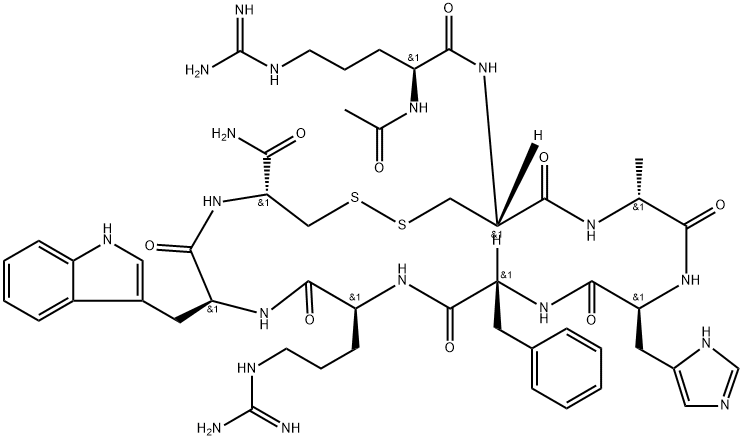
What is Irofulven?
Absorption
Setmelanotide has a Tmax of 8 hours.
Toxicity
Data regarding overdoses of setmelanotide are not readily available. In the event of an overdose, patients should be treated with symptomatic and supportive care.
Indications
Setmelanotide is indicated for chronic weight management in patients 6 years and older with obesity due to pro-opiomelanocortin (POMC) deficiency, proprotein subtilisin/kexin type 1 (PCSK1) deficiency, or leptin receptor (LEPR) deficiency as determined by an approved test demonstrating variants in POMC, PCSK1, or LEPR genes that are interpreted as pathogenic, likely pathogenic, or of uncertain significance. These conditions affect the MC4R signalling pathway.
Setmelanotide is also indicated for chronic weight management in patients 6 years and older with obesity due to Bardet-Biedl syndrome.
The drug is not reported to be effective in patients with POMC, PCSK1, or LEPR variants classified as benign or likely benign, as well as other types of obesity not listed above.
Background
Setmelanotide is the first available treatment for patients with pro-opiomelanocortin, proprotein subilisin/kexin type 1, or leptin deficiencies. It is an agonist of the melanocortin 4 receptor. Earlier attempts at agonizing MC4R (such as LY2112688) lead to successful weight loss, but also an increase in blood pressure and heart rate. Other earlier treatments for these patients included gastric bypass surgery. Patients taking setmelanotide experienced an average weight loss of 0.6 kg/week.
Imcivree was granted EMA orphan designation on November 19, 2018 and FDA approval on November 25, 2020. On May 4, 2023, it was approved by Health Canada.
Pharmacokinetics
Setmelanotide agonizes MC4R, downstream of multiple potential genetic deficiencies, to induce a feeling of satiety for chronic weight management. It has a moderate duration of action as it is given daily. Patients should be counselled regarding the risk of disturbances in sexual arousal, depression and suicidal ideation, and darkening of skin pigmentation. Exercise caution in neonates and low birth weight infants, as they may experience serious adverse effects due to benzyl alcohol.
Metabolism
Setmelanotide is expected to be metabolized to small peptides and amino acids.
Properties of Irofulven
| Density | 1.54±0.1 g/cm3(Predicted) |
| pka | 12.82±0.70(Predicted) |
Safety information for Irofulven
Computed Descriptors for Irofulven
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran

You may like
-
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 (3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
(3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
54314-85-1 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2